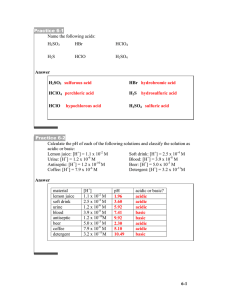
Acids and Bases
advertisement

Acids and Bases 1. Does the pair represent conjugate acid-base pair? a. HClO4, ClO4 – b. HCl, ClO– c. H2PO4–, HPO42– d. HNO3, NO3– 2. Write the dissociation equation for each of the following as they react with water and label the conjugate base. (Assume each is acting as an acid.) a. H2S b. HS– c. NH3 d. H2SO3 3. Calculate [H+] or [OH-] as required for each of the following at 25 °C and state whether the solution is neutral, acidic, or basic. a. [H+] = 3.4 x 10–4 M b. [H+] = 2.6 x 10–11 M c. [OH-] = 6.2 x 10–9 M d. [OH-] = 8.1 x 10–3 M 4. Which is greater, [OH-] or [H+], if [H+] = 2.8 x 10–5 M in an aqueous solution at 25 °C? 5. How many times greater is [OH-] than [H+] if [H+] = 1.0 x 10–9 M in an aqueous solution at 25 °C? 6. Determine the volume of 0.250 M KOH required to titrate 25.0 mL of 0.150 M HCl. 7. Determine the molarity of 72.3 mL of Ca(OH)2 if it is titrated by 125 mL of 0.450 M HCl. 8. Determine the volume of 0.934 M NaOH required to titrate 147 mL of 0.632 M H2SO4. 9. lf 26.5 mL of a 0.200 M aqueous solution of NaOH is required to titrate 50.0 mL of an aqueous solution of HNO3, what is the concentration (molarity) of the HNO3 solution?