MS Word
advertisement
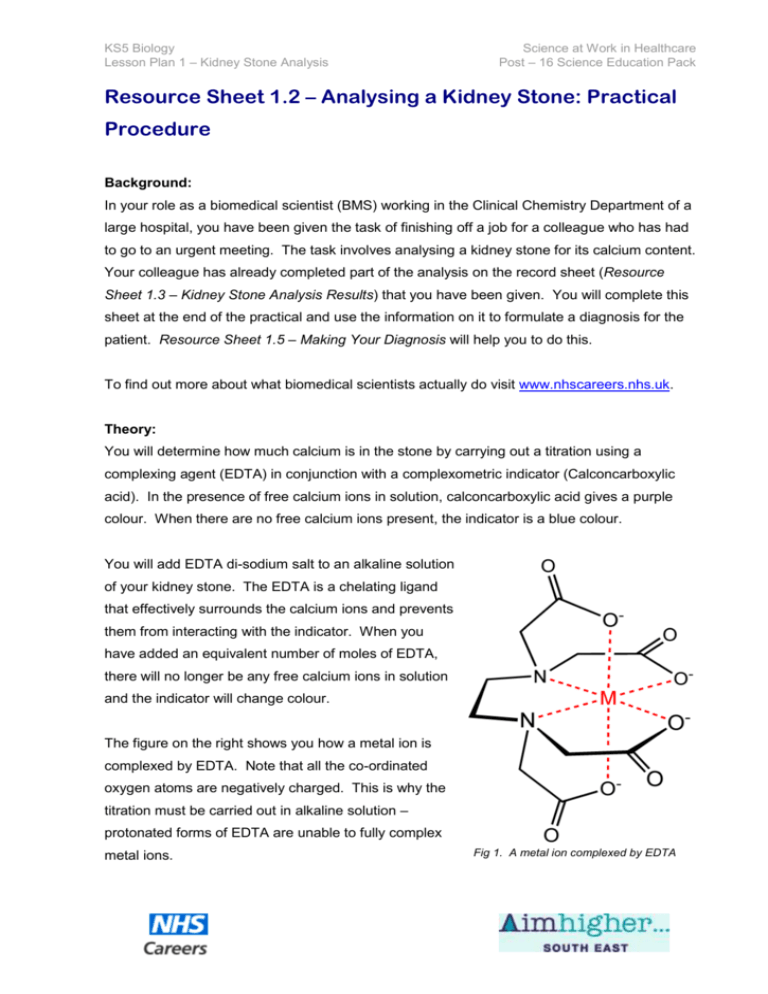
KS5 Biology Lesson Plan 1 – Kidney Stone Analysis Science at Work in Healthcare Post – 16 Science Education Pack Resource Sheet 1.2 – Analysing a Kidney Stone: Practical Procedure Background: In your role as a biomedical scientist (BMS) working in the Clinical Chemistry Department of a large hospital, you have been given the task of finishing off a job for a colleague who has had to go to an urgent meeting. The task involves analysing a kidney stone for its calcium content. Your colleague has already completed part of the analysis on the record sheet (Resource Sheet 1.3 – Kidney Stone Analysis Results) that you have been given. You will complete this sheet at the end of the practical and use the information on it to formulate a diagnosis for the patient. Resource Sheet 1.5 – Making Your Diagnosis will help you to do this. To find out more about what biomedical scientists actually do visit www.nhscareers.nhs.uk. Theory: You will determine how much calcium is in the stone by carrying out a titration using a complexing agent (EDTA) in conjunction with a complexometric indicator (Calconcarboxylic acid). In the presence of free calcium ions in solution, calconcarboxylic acid gives a purple colour. When there are no free calcium ions present, the indicator is a blue colour. You will add EDTA di-sodium salt to an alkaline solution of your kidney stone. The EDTA is a chelating ligand that effectively surrounds the calcium ions and prevents them from interacting with the indicator. When you have added an equivalent number of moles of EDTA, there will no longer be any free calcium ions in solution and the indicator will change colour. The figure on the right shows you how a metal ion is complexed by EDTA. Note that all the co-ordinated oxygen atoms are negatively charged. This is why the titration must be carried out in alkaline solution – protonated forms of EDTA are unable to fully complex metal ions. Fig 1. A metal ion complexed by EDTA KS5 Biology Lesson Plan 1 – Kidney Stone Analysis Science at Work in Healthcare Post – 16 Science Education Pack Safety: Wear gloves at all times, and wash your hands if any chemicals come into contact with them. Wear safety glasses and a lab coat at all times. The procedure you will follow is adapted from a genuine NHS standard operating procedure Procedure: 1) Place your kidney stone in a mortar and grind to a fine powder using a pestle. 2) Accurately weigh about 0.100g of the powder into a plastic weighing boat using a 3.d.p balance (if available) and record the mass on Resource Sheet 1.3. 3) Add 5 drops of concentrated sulphuric acid to the powder and mix thoroughly using a spatula. Conc. Sulphuric acid is highly corrosive. Take care! 4) Using distilled water from a wash bottle, carefully wash the mixture in the weighing boat into a clean 500 cm3 volumetric flask. Make sure all the material in the weighing boat and on the spatula is transferred to the flask. 5) Fill the volumetric flask up to the mark with distilled water, and shake vigorously. (Don’t be concerned if there is still a little undissolved solid in the bottom). 6) Using a 10 cm3 transfer pipette, remove 10cm3 of the solution from the volumetric flask and place it in a 100 cm3 conical flask. 7) Using a Pasteur pipette, add 3 or 4 drops of the calcon indicator solution to the solution in the conical flask. 8) Add 2 or 3 drops of 25% sodium hydroxide solution to the solution in the flask. (The solution should have a purple colour) 9) Fill a 50 cm3 burette with 0.001 mol dm-3 EDTA di-sodium salt solution. 10) Carry out an ‘overshoot’ titration to find out roughly how much EDTA solution is required to complex all of the calcium ions in solution. (The end point is when the colour of the solution turns from purple to permanent blue*) 11) Repeat steps 6-9, then carry out a titration to accurately determine how much EDTA solution is required to complex all of the calcium ions in 10 cm3 of solution. 12) Record results on Resource Sheet 1.3 and repeat step 11 until you get concordant results. 13) Use your results to calculate what percentage of the stone (by mass) is calcium. Record your result on the card you were given by your teacher. 14) Use the results on Resource Sheet 1.3 and the information on Resource Sheet 1.5 to suggest a diagnosis for the patient who provided your kidney stone. * The end point is difficult to see in this titration. It might be worth setting up a reference sample containing EDTA.2Na+ solution and a few drops each of Calcon indicator solution and 25% NaOH solution to show you the colour you are aiming for at the end point.









