Lipids

Lipids
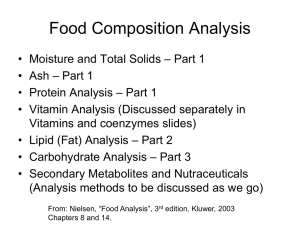
Lipids are composed of:
• Carbon
• Hydrogen
• Oxygen
“CHO”
BUT: Lipid molecules have a larger number of Carbon and Hydrogen and a smaller number of Oxygen than Carbohydrates
Properties of Lipids
• Fats, oils, and waxes are lipids.
• Unlike the other organic compounds, lipids do not dissolve in water; therefore, they are nonpolar or insoluble.
Lipid Functions
• Lipid molecules provide a barrier b/t a cell and its watery environment
Lipid Functions
• Insulation of whales and walruses
Lipid Functions
• Skin oils help repel water from feathers of ducks
Lipid Functions
• Waxy layer of plants, keeps water in
Fatty Acids
• Lipids are composed of building blocks or
MONOMERS called Fatty Acids.
Fatty Acids
• Carboxyl end (-COOH)
– polar
– Hydrophilic: “water loving”
– Hydrocarbon end (CH
3
)
• Non-polar
• Hydrophobic: “Water fearing”
WATER
Arrangement of FA in Cell
Functions of Lipids
• Store Energy!!
• Fats have more H bonds than Carbs and can store more energy.
Kinds of LIPIDS
1. Triglycerides
2. Waxes
3. Steriods
4. phospholipds
Triglycerides
Composed of:
1. 1 glycerol molecule
2. 3 molecules of fatty acids
TWO categories of triglycerides
1. Saturated
2. unsaturated
• Plant waxes
– Forms protective coating on outer surface
Waxes
• Earwax
– Prevents bacteria from entering middle ear
Steroids
• Composed of 4 carbon rings
• Found :
– Hormones
– Toad venom
– Plant poisons
– Chlorophyll
– Cholesterol
Steroids