Oxford Radcliffe Hospitals Research Protocol Amendments Document Ref. No.: RD004
advertisement
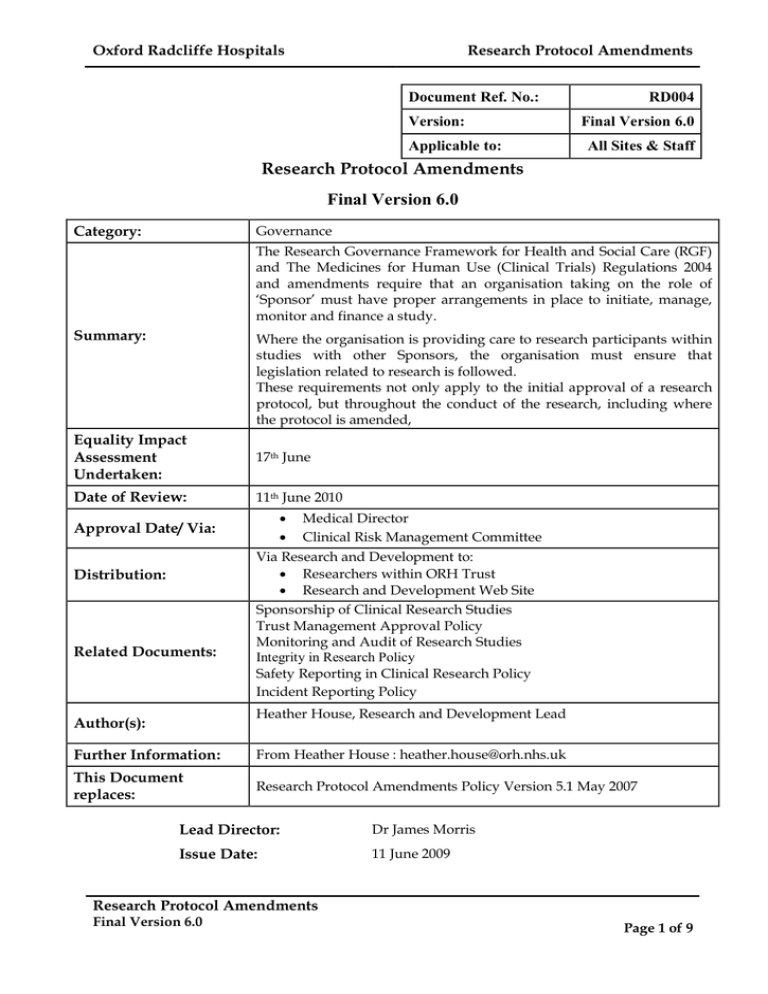
Oxford Radcliffe Hospitals Research Protocol Amendments Document Ref. No.: Version: Applicable to: RD004 Final Version 6.0 All Sites & Staff Research Protocol Amendments Final Version 6.0 Category: Governance The Research Governance Framework for Health and Social Care (RGF) and The Medicines for Human Use (Clinical Trials) Regulations 2004 and amendments require that an organisation taking on the role of ‘Sponsor’ must have proper arrangements in place to initiate, manage, monitor and finance a study. Summary: Where the organisation is providing care to research participants within studies with other Sponsors, the organisation must ensure that legislation related to research is followed. These requirements not only apply to the initial approval of a research protocol, but throughout the conduct of the research, including where the protocol is amended, Equality Impact Assessment Undertaken: 17th June Date of Review: 11th June 2010 Approval Date/ Via: Distribution: Related Documents: · Medical Director · Clinical Risk Management Committee Via Research and Development to: · Researchers within ORH Trust · Research and Development Web Site Sponsorship of Clinical Research Studies Trust Management Approval Policy Monitoring and Audit of Research Studies Integrity in Research Policy Safety Reporting in Clinical Research Policy Incident Reporting Policy Heather House, Research and Development Lead Author(s): Further Information: From Heather House : heather.house@orh.nhs.uk This Document replaces: Research Protocol Amendments Policy Version 5.1 May 2007 Lead Director: Dr James Morris Issue Date: 11 June 2009 Research Protocol Amendments Final Version 6.0 Page 1 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments C O N T E N T S Page Introduction…………………………………………………………………………………….... 3 Policy Statement………………………………………………………………………………..... 3 Aim…………………………………………………………………………………………….... 3 Scope……………………………………………………………………………………………... 3 Definitions……………………………………………………………………………………….. 3 Organisational Responsibilities………………………………………………………………. 5 Chief Investigator…………………………………………………………………………… 5 Principal Investigator…...…………………………………………... 5 Research and Development Staff …………………………………………………………... 5 Research & Development Lead…………… 5 Organisational Arrangements…………………………………………………………………. 5 Education and Training………..…………………………………………………………… 5 Implementation Guidance…………………………………………………………………... 5 Equality Impact Assessment……………………………………………………………….. 5 Monitoring and Review……………………………….....……………………………..... 6 References…………………………………………………………………………………… 6 Document History………………………………………………………………………….. 6 Appendix A: Implementation Plan…………………………………………………………… 7 Urgent Safety Measures 7 Identification of Requirement for Protocol Amendment 7 Submission Timelines 7 Documents Required 7 Review Process 7 Non­substantial Amendments 7 Implementation 8 Monitoring the Progress of Research Studies 8 Appendix B: List of Abbreviations 9 Research Protocol Amendments Final Version 6.0 Page 2 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments Research Protocol Amendments Introduction 1. The Research Governance Framework for Health and Social Care (RGF) and The Medicines for Human Use (Clinical Trials) Regulations 2004 and amendments require that an organisation taking on the role of ‘Sponsor’ must have proper arrangements in place to initiate, manage, monitor and finance a study. 2. Where the organisation is providing care to research participants within studies with other Sponsors, the organisation must ensure that legislation related to research is followed. by the researchers. 3. These requirements apply not only to the initial approval of a research protocol, but also throughout the conduct of the research, including where the research protocol is amended, Policy Statement 4. It is the policy of the Trust to: 4.1 Protect the safety, dignity, rights and well­being of all patients involved in clinical research. 4.2 Ensure that arrangements are in place for the management and monitoring of research studies, where the Trust has taken on the role of Sponsor, including compliance with the relevant regulations. Aim 5. This policy sets out a consistent procedure for the review and authorising of amendments to the protocol of research studies for which the Oxford Radcliffe Hospitals NHS Trust (‘the Trust’) has been asked to take on the role of ‘Sponsor’ or host institution. 6. This procedure aims to ensure that the Trust takes responsibility for the ongoing quality of research studies. Scope 7. This Policy applies to anyone conducting research within the Trust, whether such research is sponsored or hosted by ORH. Definitions Sponsor 8. The organisation taking responsibility for initiation, management and financing (or arranging the financing) of a clinical trial of an investigational medicinal product (CTIMP) or research study. Substantial Protocol Amendment 9. A protocol amendment is required if it is necessary to make changes to the research after approvals from the Research Ethics Committee (REC), the Medicines and Healthcare products Regulatory Agency (MHRA) (for CTIMPS) and Research and Development (R&D) Department have been received. The REC, MHRA and relevant R&D department Research Protocol Amendments Final Version 6.0 Page 3 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments must approve substantial amendments, before they are implemented, unless the amendment requires immediate implementation due to safety concerns, in which case, a formal amendment must subsequently be submitted. A substantial amendment is defined as an amendment to the terms of the protocol or any other supporting documentation that is likely to affect to a significant degree: § the safety or physical or mental integrity of the subjects of the trial § the scientific value of the trial § the conduct or management of the trial § the quality or safety of any investigational medicinal product used in the trial Further guidance is available at: www.nres.npsa.nhs.uk/applicants/after­ethical­review/amendments/#Examples Non­substantial Protocol Amendment 10. These can be defined as changes to the details of the study which have no significant implications for the subjects, conduct, management, or the scientific value of the study. Urgent Safety Measure 11. An amendment which needs to be implemented as a matter of urgency, in order to protect research participants against any immediate hazard to their health or safety. Chief Investigator 12. The individual, as identified in the ethics application, who takes overall for the conduct of a clinical study. responsibility Principal Investigator 13. The individual who takes on responsibility for conduct of the study at a particular site. Clinical Trial of Investigational Medicinal Product (CTIMP) 14. Any investigation in human subjects, other than a non­interventional trial*, intended a) To discover or verify the clinical, pharmacological or other pharmacodynamic effects of one or more medicinal products, b) To identify any adverse reactions to one or more medicinal products or c) To study the absorption, distribution, metabolism and excretion of one or more such products With the object of ascertaining the safety or efficacy of those products Such trials will be governed by the Medicines for Human Use (Clinical Trials) Regulations 2004 and updates. Clinical Research Study 15. Any investigation in human subjects, which may involve an intervention to their normal clinical care, or may be purely observational. Research Protocol Amendments Final Version 6.0 Page 4 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments Organisation Responsibilities Chief Investigator (CI) 16. Identify the need for an amendment. Principal Investigator (or delegate) 17. Communicate with R&D, providing all relevant documentation for approval of the amendment. Research and Development Staff 18. Provide advice and information to investigators regarding the amendment process. Collation of all relevant documentation. Appraisal of any impact the amendment may have on the Trust. Research & Development Lead 19. Authorise ongoing Trust Management Approval with regard to the amendment. Organisational Arrangements Education & Training 20. All staff involved in the conduct of clinical trials will undertake training in Good Clinical Practice GCP) prior to beginning their involvement in a trial. The process of submission of substantial amendments is covered within this training. Implementation Guidance 21. Attached as Appendix A is an Implementation Plan, which details the measures to be undertaken to ensure that the requirements of this policy are fully integrated in day to day operations. Equality Impact Assessment 22. In accordance with Equality & Diversity legislation, this Policy has had an Equality Impact Assessment undertaken. It has been determined that this Policy does not discriminate against any individual or group and a full copy of the assessment can be viewed on the Research and Development intranet page. Monitoring & Review 23. Staff of the Trust R&D Department are responsible for auditing compliance with the policy on an annual basis. The R&D Manager/Lead will be responsible for formulation and implementation of remedial action, should this be required. 24. The Trust R&D Department will monitor and update this policy to reflect changes in legislation and examples of best practice. Research Protocol Amendments Final Version 6.0 Page 5 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments References 25. The Medicines for Human Use (Clinical Trials) Regulations 2004 and Amendments 26. The Department of Health Research Governance Framework for Health and Social Care 2005 27. ICH Harmonised Tripartite Guideline for Good Clinical Practice. Document History Version Date Author Status Comment 5 Feb 2006 E Tucker/J Allen Superseded Reviewed and updated 5.1 May 2007 J Allen Superseded Reviewed and updated Research Protocol Amendments Final Version 6.0 Page 6 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments Appendix A: Implementation Plan Urgent Safety Measures 28. If the amendment requires urgent safety measures to be taken, it should be implemented immediately. A formal amendment must subsequently be submitted. For CTIMPS, any urgent safety measures must be notified to the MHRA within three days. Identification of Requirement for Protocol Amendment 29. Researchers should view the guidance for submitting amendments at the following web addresses: National Research Ethics Service (NRES) http://www.nres.npsa.nhs.uk/ Medicines and Healthcare products Regulatory Agency (MHRA) http://www.mhra.gov.uk/Howweregulate/Medicines/Licensing/Licensingofmedicin es/Clinicaltrials/index.htm Gene Therapy Advisory Committee (GTAC) http://www.advisorybodies.doh.gov.uk/genetics/gtac/ Submission Timelines 30. Submission of a Substantial Amendment to the REC, MHRA (where applicable) and R&D can be undertaken in parallel. R&D staff can process the amendment in anticipation of the relevant approvals being granted, providing that the approval letters are, ultimately provided. Documents Required 31. All documents submitted to the Research Ethics Committee, should also be provided to R&D. Other than the approval letters, an amendment cannot be processed by R&D until all documentation has been provided. Review Process 32. On receipt of the required documents, a member of the R&D Team will collate all relevant documents and assess whether there is any impact on the Trust and whether the amendment is compliant with the relevant legislation. Further information may be requested, where anything is unclear. 33. Once the final approval letters relating to the amendment have been provided, a letter, confirming ongoing Trust Management Approval (TMA) will be prepared and will be signed by the R&D Lead or other authorised signatory. The original copy will be supplied to the Investigator. Non­substantial Amendments 34. Non­substantial amendments should be copied to R&D for information and to update the R&D database. Research Protocol Amendments Final Version 6.0 Page 7 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments Implementation 35. Other than urgent safety measures, protocol amendments should not be implemented until TMA has been receeived. Monitoring the Progress of Research Studies 36. The progress of the study through the approvals process is monitored through on­going contact with the investigator. Copies of correspondence between the ethics committee and the investigator, and between the MHRA (for CTIMPS) and the investigator, may also be forwarded to R&D. The R&D database will be promptly updated on receipt of any information. Research Protocol Amendments Final Version 6.0 Page 8 of 9 Oxford Radcliffe Hospitals Research Protocol Amendments Appendix B: List of Abbreviations CTIMP Clinical Trial of an Investigational Medicinal Product GCP Good Clinical Practice GTAC Gene Therapy Advisory Committee MHRA Medicines and Healthcare products Regulatory Agency NRES National Research Ethics Service REC Research Ethics Committee R&D Research and Development RGF The Research Governance Framework for Health and Social Care TMA Trust Management Approval Research Protocol Amendments Final Version 6.0 Page 9 of 9