Predicting Capacity of Hard Carbon Anodes in Sodium-Ion Batteries
advertisement

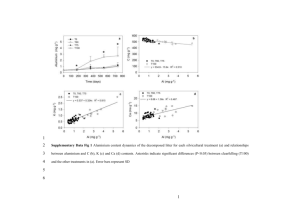
Predicting Capacity of Hard Carbon Anodes in Sodium-Ion Batteries Using Porosity Measurements Bommier, C., Luo, W., Gao, W. Y., Greaney, A., Ma, S., & Ji, X. (2014). Predicting capacity of hard carbon anodes in sodium-ion batteries using porosity measurements. Carbon, 76, 165-174. doi:10.1016/j.carbon.2014.04.064 10.1016/j.carbon.2014.04.064 Elsevier Accepted Manuscript http://cdss.library.oregonstate.edu/sa-termsofuse Predicting Capacity of Hard Carbon Anodes in Sodium-Ion Batteries Using Porosity Measurements Clement Bommier1, Wei Luo1, Wen-Yang Gao3, Alex Greaney2, Shengqian Ma3 and Xiulei Ji1 1. Department of Chemistry, Oregon State University, Corvallis, Oregon, 97331, USA 2. Department of Mechanical, Industrial and Materials Engineering, Oregon State University, Corvallis, Oregon, 97331, USA 3. Department of Chemistry, University of South Florida, Tampa, Florida, 33620, USA Abstract We report an inverse relationship between measurable porosity values and reversible capacity from sucrose-derived hard carbon as an anode for sodium-ion batteries (SIBs). Materials with low measureable pore volumes and surface areas obtained through N2 sorption yield higher reversible capacities. Conversely, increasing measurable porosity and specific surface area leads to sharp decreases in reversible capacity. Utilizing a low porosity material, we thus are able to obtain a reversible capacity of 335 mAh g-1. These findings suggest that sodium-ion storage is highly dependent on the absence of pores detectable through N2 sorption in sucrosederived carbon. Corresponding author: Tel: +1 541-737-6798, E-mail: david.ji@oregonstate.edu 1 1. Introduction In the coming years, research and development of new energy technologies will be an essential part of meeting rising electricity demand while offsetting growing environmental concerns. There are two concurrent priorities in this effort: a drive towards renewable energy generation combined with an inexpensive and large-scale electricity energy storage (EES). The state-of-the-art EES system currently available is lithium-ion batteries (LIBs). [1] Though they offer high reversibility and efficiency, they are also very expensive due to the rarity and high extraction costs of lithium metal. This is where sodium-ion batteries (SIBs) present themselves as a feasible alternative.[2] They share many similar properties with LIBs, but are much more economically advantageous due to the low cost of sodium, as it is the 6th most abundant element in Earth’s crust and can easily be mined from abundant sources. This renders it 95% cheaper than Lithium. [3] However, the size difference between the two ions, 95 pm for Na+ versus 68 pm for Li+, makes it such that the technologies of LIBs and SIBs are not always interchangeable. This is especially true when considering the anode material. Unlike LIBs, SIBs cannot use graphitic materials for anodes. It has been well documented that intercalation of Na+ in graphite is unfavorable.[4,5] Whereas in the rocking-chair model of LIBs, the graphitic anode possesses the chemical formula of LiC6 after intercalation, its SIB counterpart has a chemical formula of NaC70.[6] This has led to significant efforts aimed towards developing a suitable SIB anode. A variety of options have been explored, including carbon materials,[7,8,9,10] phosphorous,[11,12] organic compounds,[13] metal oxides,[14,15,16] metal nitrides,[17] sodium ternary compounds,[18,19,20,21,22] thin film anodes[23] as well as various types of alloys.[24,25,26,27,28,29,30] Of the above candidates, carbon-based materials have distinguished themselves as a promising solution as they are cheap, easily attainable and non-toxic. Various types have been investigated such as carbon black,[31] carbon microspheres,[32] hierarchically porous carbon,[33] nitrogen-doped carbons[34,35] graphene nanocomposites[36] and nanostructures.[37,38] While all 2 these materials have their various merits, much interest still remains in the research disordered graphitic carbon—otherwise known as hard carbon. In previous studies, hard carbon has demonstrated a stable cycling capacity of 285 mAh g-1 and 300 mAh g-1 by Alcántra et al.[31] and Dahn et al.[10], respectively, and has lately managed to surpass 300 mAh g-1 for over 100 cycles as reported by Palacin et al.[39] The widely-accepted theory suggests that hard carbon can effectively store sodium ions in the nanopores that stem from its randomly scattered graphene nanodomains — this is otherwise known as the ‘falling-card’ model.[9] This concept leads us to investigate whether a greater nanoporosity will be more effective for sodium-ion storage, which would thus result in a higher reversible capacity. Herein, we demonstrate a model that strongly suggests reversible capacity of SIBs is inversely proportional to increases in both pore volume and surface area of the hard carbon active material. By reducing the measurable porosity, we report one of the highest reversible capacities to date of hard carbon at 335 mAh g-1. 2. Experimental Section 2.1. Materials Synthesis and Characterizations The active hard carbon material used in this study is derived from sucrose (Macron Chemicals). Sucrose is dehydrated for 24 hrs under atmospheric conditions at 180 ºC. Chunks of the resulting material are pyrolysed under continuous argon gas flow in a tube furnace for 6 hrs. The pyrolysis was done at 800 ºC, 900 ºC, 1000 ºC and 1100 ºC, with a heating rate of 5 ºC min-1. CO2 activation was performed on samples pyrolysed at 1000 ºC at temperatures of 700 ºC, 800 ºC and 900 ºC for durations of 1 hr, 2 hrs, 5 hrs and 10 hrs with a heating rate of 10 ºC min-1 and a CO2 flow rate of 100 cm3 min-1. Following the synthesis of the hard carbon material, it was characterized with X-ray diffraction (XRD) using a Rigaku Ultima IV Diffractometer operating at 40 kV and 40 mA using Cu Kα radiation (λ = 1.5406 Å) and a 3 WITec confocal Raman Spectroscopy with a 514 nm laser. Surface morphology was obtained through a FEI Quanta 600 SEM. Surface area was obtained through the Brunnauer-EmmettTeller model (BET) and pore volume was obtained through Density Functional Theory (DTF) using N2 sorption with a Micromeritics ASAP 2020 Surface Area Analyzer. Elemental analyses were performed on the samples pyrolysed at different temperatures by the Environmental Division of ALS Global. 2.2. Electrochemical Measurements Electrodes were made using a 7:2:1 weight ratio of hard carbon, Super-P carbon black (TimCal) and poly-1,1-difluoroethene (PVDF) binder, respectively. This was mixed in an nmethyl-2-pyrrodilinone (NMP) solvent before being doctor-bladed onto copper foil. Once the anodes were prepared they were punched into disks with loading masses typically between 1.5 to 1.8 mg cm-2 which were then vacuumed and moved into in an argon-filled glove box for coin cell assembly. The coin cells were composed of a solid sodium metal counter electrode, a glass-fiber separator (PALL) and a 1.0 M NaClO4 electrolyte solution dissolved in a 1:1 weight mixture of ethylene carbonate / propylene carbonate (EC/PC). The batteries were galvanostatically cycled on an Arbin BT2000 system at an initial current rate of 40 mA g-1, which translates into a cycling rate of C/8 as we consider 300 mAh g-1 to be 1 C. Higher rates from 100 mA g-1 to 1 A g-1 were used to investigate the rate capability of the materials. At a current rate of 40 mA g-1 carbon black has a reversible capacity of 150 mAh g-1, which is included in the supplementary section (Figure S1). Since our electrodes comprise 20 weight% of carbon black, this would represent a capacity contribution of 30 mAh g-1. Being on the side of caution, we removed 40 mAh g-1 of capacity at a current rate of 40 mA g-1, which gives us the reversible capacity of 335 mAh g-1. 4 Cyclic voltammetry was performed on a VMP-3 multi-channel workstation at a scanning rate of 0.2 mV s-1 at room temperature. Statistical modeling data was obtained through the R programming package. 3. Results and Discussion We pyrolysed sucrose at 1000 ºC for preparation of the hard carbon. We first tested whether increasing porosity via CO2 activation would lead to higher capacities. The CO2 activation can be described by the following reaction: Table 1. Measurable pore volume/surface area data and capacities for samples obtained through CO2 activation. CO2 Activation Temperature/ Time untreated untreated 900 ºC-1hr 900 ºC-1hr 900 ºC-2hrs 900 ºC-2hrs 900 ºC-5hrs 900 ºC-5hrs 900 ºC-10hrs 900 ºC-10hrs 800 ºC-1hr 800 ºC-1hr 800 ºC-2hrs 800 ºC-2hrs 800 ºC-5hrs 800 ºC-5hrs 800 ºC-10hrs 800 ºC-10hrs 700 ºC-1hr 700 ºC-1hr 700 ºC-2hrs 700 ºC-2hrs 700 ºC-5hrs 700 ºC-5hrs 700 ºC-10hrs 700 ºC-10hrs DFT Pore Volume (cm3 g-1) BET Surface Area (m2 g-1) abs(ln DFT Pore Volume) ln Surface Area 1st cycle Reversible Capacity (mAh g-1) 0.042 0.042 0.177 0.177 0.265 0.265 0.607 0.607 0.908 0.908 0.082 0.082 0.126 0.126 0.123 0.123 0.197 0.197 0.122 0.122 0.094 0.094 0.099 0.099 0.140 0.140 58.7 58.7 219.6 219.6 340.6 340.6 832.5 832.5 1410.3 1410.3 102.6 102.6 154.4 154.4 155.4 155.4 256.9 256.9 152.1 152.1 126.4 126.4 141.9 141.9 196.5 196.5 3.16 3.16 1.73 1.73 1.33 1.33 0.50 0.50 0.10 0.10 2.51 2.51 2.07 2.07 2.09 2.09 1.62 1.62 2.10 2.10 2.37 2.37 2.31 2.31 1.97 1.97 4.07 4.07 5.39 5.39 5.83 5.83 6.72 6.72 7.25 7.25 4.63 4.63 5.04 5.04 5.05 5.05 5.55 5.55 5.02 5.02 4.84 4.84 4.95 4.95 5.28 5.28 282.9 290.4 239.3 214.9 208 186.5 107.1 120.7 49.3 43.6 257.4 229.6 240.6 236.6 221.7 218.5 268.3 228.9 232.5 225.3 205.8 192.7 263.5 255.1 249.8 241.5 5 Irreversible Capacity (%) 28.6% 21.0% 33.5% 22.2% 45.0% 54.9% 71.9% 75.7% 80.8% 89.9% 29.1% 32.9% 31.2% 32.0 23.9% 44.0% 28.9% 37.0% 25.6% 34.3% 32.7% 45.8% 21.4% 29.5% 25.1% 25.3% This reaction consumes part of the solid carbon, thereby increasing its porosity and surface area. Samples were initially activated at a temperature of 900 ºC, with reaction durations of 1 hr, 2 hrs, 5 hrs and 10 hrs (Table 1). Half cells were then made from the activated carbons and characterized by galvanostatic charge/discharge cycling (Figure 1a). The capacities reported are those obtained during the desodiation process of the anode, often referred to as reversible capacity, and are solely attributed to the sucrose-derived hard carbon. An activated sample is referred to as ‘T ºC-n hrs’ if it was treated at T ºC for n hours. Figure 1. Initial cycling performance at 40 mA g-1 for carbons activated a) 900 ºC b) 800 ºC c) 700 ºC for differernt durations and d) Initial cycling performance of carbons pyrolysed at different temperatures. The unactivated sample has an initial reversible capacity of 290 mAh g-1. After activation of 1 hr and 2 hrs, the capacities drop to 239 mAh g-1 and 208 mAh g-1, respectively. The samples that were activated for 5 hrs and 10 hrs exhibit even lower reversible capacities of 120 mAh g-1 and 49 mAh g-1, respectively. It is evident that CO2 activation at 900ºC decreases reversible capacity, with longer activation durations yielding progressively worse results. We hoped that milder activation temperatures of 700 ºC and 800 ºC would be more 6 conducive to forming the desired nanopores, and offer an improvement over those obtained at 900 ºC. However, these lower temperatures also caused a decrease in reversible capacity (Figure 1b, c). Interestingly, longer activation durations at lower temperatures do not necessarily lead to lower capacities. Contrary to what had been observed at 900 ºC, the 700 º C-2 hrs activated carbon shows an initial reversible capacity of 206 mAh g-1, the lowest of the 700 ºC series, while the 700 ºC-5 hrs has the highest reversible capacity at 264 mAh g-1. We do not understand this phenomenon so far. Figure 2. XRD patterns of different samples. a) Samples after CO2 activation at 900 ºC for different durations. b) Samples of 700 ºC-10h and 800 ºC-10h. The untreated sample in a) and b) is the hard carbon pyrolysed at 1000 ºC. c) Sucrose-derived hard carbon pyrolysed at different temperatures, unactivated. Seeing losses in capacity following CO2 activation led us to search for a link between the physical properties of the materials and their sodium-ion storage performance. XRD patterns and Raman spectra were collected for the untreated carbon and its CO2 activated counterparts (Figure 2a,b and Figure S2). All the samples obtained demonstrate the typical 7 characteristics of amorphous carbon: broad peaks at 24º and 43º, representing the (002) and (100) planes, respectively. The 900 ºC-5 hrs and 900 ºC-10 hrs samples exhibit even less resolved XRD peaks, indicating a disruption of the amorphous structure by activation. Raman spectra were inconclusive as well. The intensity ratios (Id/Ig) of the D-band (1350 cm-1) representing the defected sp2 hybridized carbon over the G-band (1580 cm-1) measuring the sp2 hybridized carbon show little variation (Table S1).An Id/Ig greater than 1 indicates a more disordered carbon. On the other hand, Density Functional Theory (DFT) pore volume and BrunauerEmmett-Teller (BET) surface area, calculated through N2 sorption measurements, show dramatic changes following CO2 activation. All activated samples exhibit greater measurable pore volume and surface area than the untreated control. The roughened surface morphology is evident from the SEM images (Figure 3a,b). Higher CO2 activation temperatures and/or longer reaction times give rise to larger pore volume and surface area. For example, the 900 º C-10 hrs sample yields a surface area of 1410.3 m2 g-1 and a pore volume of 0.91 cm3 g-1, comparing to a pore volume of 0.042 cm3 g-1 and a surface area of 58.7 m2 g-1 for the unactivated material (Table 1). Figure 3. SEM images showing that activation roughens surface morphology of sucrosederived carbon a) pyrolysed at 1000 ºC, unactivated, b) pyrolysed at 1000 ºC and activated under CO2 at 900 ºC for 10 hrs. mesopores (2 nm-50 nm) and micropores (less than 2 nm) are not observable at such low magnification. 8 (a) (b) Figure 4. Linear and natural logarithm transformed models plotting the reversible capacity in the 1st cycle as a function of a) DFT specific pore volume b) BET specific surface area. The best and worst results for the same materials were plotted. We then plotted the first reversible capacity of all the samples as a function of their measurable specific pore volume and surface area (Figure 4). When a linear regression 9 analysis was performed, the data yields coefficient of determination values (R2) of 0.889 for the pore volume plot and of 0.879 for the surface area plot. To minimize the bias from the outlying data points from 900 ºC-5hrs and 900 ºC-10hrs, while spreading out the clustered ones, we performed a natural logarithm (ln) transform of the pore volume and surface area values. As the pore volumes were quantities smaller than one for all samples, we further took the absolute value (abs) to avoid negative numbers. The consequent R2 values obtained are 0.793 and 0.810 for the pore volume and surface area plots, respectively. Both models demonstrate that reversible capacity is inversely proportional to DFT pore volume and surface area. Following the CO2 activation, we tried to gauge the effect that different sucrose pyrolysis temperatures would have on the active material. For this, we utilized temperatures of 800 ºC, 900 ºC and 1100 ºC compared to the 1000 ºC used for our original material. Once again, the reversible capacities are closely related to porosity values. The material obtained at 1100 ºC has the lowest measurable pore volume and surface area, 0.015 cm3 g-1 and 24.8 m2 g-1, of the all samples obtained. It yields an initial reversible capacity of 335 mAh g-1 with the contribution of super-P carbon taken out. Conversely, the sample pyrolysed at 800 º C has the highest pore volume and surface area of the series at 0.199 cm3 g-1 and 265.8 m2 g-1, respectively, and only achieved a reversible capacity of 200 mAh g-1 (Table 2). When the data points from the different pyrolysis temperatures were added to the plots, the R2 values stay relatively constant: 0.862 for the DFT Pore Volume model and 0.844 for the Surface Area model. The R2 for the ln transformed models is also unchanged, standing at 0.786 for the ln DFT Pore Volume model and 0.816 for the ln Surface Area model (Figure 4). Looking at the results of the regression analyses performed, all of the p-values obtained, with 30 degrees of freedom (DF), are below 1.5∙10-11. A p-value is the probability of obtaining a test statistic (e.g. R2) equal to or greater than one obtained at random. A p-value of less than 10 0.05 is considered statistically significant. This proves that there is very strong evidence linking reversible capacity to DFT Pore Volume and Surface Area (Figure S3). Table 2- Measureable pore volume/surface area data and capacities for samples obtained at different pyrolysis temperatures. Pyrolysis Temperature 800 ºC 800 ºC 900 ºC 900 ºC 1000 ºC 1000 ºC 1100 ºC 1100 ºC DFT Pore Volume (cm3 g-1) BET Surface Area (m2 g-1) abs(ln DFT Pore Volume) 0.199 0.199 0.069 0.069 0.042 0.042 0.015 0.015 265.8 265.8 92.8 92.8 58.7 58.7 24.8 24.8 1.61 1.61 2.68 2.68 3.16 3.16 4.19 4.19 ln Surface Area 1st cycle Reversible Capacity (mAh g-1) 5.58 5.58 4.53 4.53 4.07 4.07 3.21 3.21 199.8 221.4 260.9 248.2 282.9 290.4 335.0 299.0 Irreversible Capacity (%) 34.0% 23.4% 24.0% 14.3% 28.6% 21.0% 4.4% 20.3% We are aware that it is possible that impurities in pyrolysed carbon can have an impact on the Na+ ion storage. Elemental analyses for the carbons obtained at different pyrolysis temperatures were conducted. All the materials tested have carbon ranging from 93 to 97 mass % and hydrogen from 0.2 to 0.6 mass % with the rest as oxygen (Table S2). Our data are expected as it was reported that pyrolysis of cellulose, as a polymer of sucrose, occurs well below 800 ºC with mainly carbon as the composition.[40] It is evident now the very much different capacities from the materials pyrolysed at various temperatures were obtained at the similar levels of extraneous hydrogen and oxygen residues. Such a vast capacity variations can be only attributed to the porosity differences between these samples. As was the case with the CO2 activation samples, the XRD profiles and Raman spectroscopy data obtained from the samples pyrolysed at different temperatures varied little. The XRD patterns all exhibit the (002) and (100) peaks, while the Id/Ig ratios obtained from Raman spectra range narrowly from 0.97 to 1.04 (Figure 2c, Figure S2, and Table S1). 11 Figure 5. Reversible capacity vs. logit transform of the percentage of the unexposed carbon atoms. The logit transform takes the formula of: This allows for a greater spread of percentage values on the x-axis. Considering that N2 can only be adsorbed in pores approximately equal to or larger than ~ 0.4 nm,[41] we can infer from the models that Na+-ion storage in hard carbon is highly dependent on the absence of such open pores. This leads us to classify the carbon atoms that contribute to the surface area and porosity as ‘exposed carbon atoms’. The percentage of ‘exposed carbon atoms’ is available through the equation ⁄ ⁄ where At represents the measured BET surface area, and r is the radius of a carbon atoms; MM and NA, are the Molar Mass and Avogadro’s number, respectively. We assume that an exposed carbon atom contributes to measurable surface area with a single hemisphere, whose area is equal to 2πr2 with r equal to 0.73 Å, the half of the bond length of adjacent carbon atoms in the graphene sheet. We can estimate ratio between the exposed carbon atoms in numerator and the total number of atoms in the denominator. 12 We then used this to calculate for the percentage of unexposed carbon atoms by using the equation Using this metric it was found that the material exhibiting the lowest reversible capacity had only 8.7% of unexposed carbon atoms, while the best performing material had 98.4% unexposed carbon atoms. Plotting the values of reversible capacity vs. Logit transform of the percentage of the unexposed carbon atoms yields an R2 value of 0.89 with a p-value of 6.3∙1016 on 30 DF (Figure 5 & Figure S4). This suggests that, unlike unexposed atoms, exposed carbon atoms have inferior contributions towards Na+-ion storage. This may be due to the fact that the exposed carbon atoms tend to act as nucleation sites for solid electrolyte interphase (SEI), a passivation layer that stems from the decomposition of the electrolyte solvents or salts. This passivation layer may disable the Na+-ion storage on the electrode surface due to its electronically-insulating nature and block the access of Na+ ions to the enclosed voids inside the carbon structure. Figure 6. A comparison of Nyquist plots of electrochemical impedance spectroscopy results of high (1410 m2 g-1) and low (24 m2 g-1) surface area carbon materials as electrodes. The measurements were taken ex-situ at various stages during the first discharge. The plots at the same voltages from high-surface-area and low-surface-area electrodes have been superimposed for clarity. 13 Evidence of the greater SEI formation on high surface area material can be seen through a comparison of electrochemical impedance spectroscopy (EIS) measurements. These were taken at various voltages in the course of the initial discharge. The low-surface-area carbon (24 m2 g-1), referred to as lowSAC, has the larger initial semi-circle, indicating a higher resistivity for interface charge transfer. However, once the batteries have been fully discharged at 0.01 V, the high surface area carbon (1410 m2 g-1), referred to as highSAC, exhibits a much larger semi-circle than the lowSAC (Figure 6). Further proof of the difference in SEI formation is seen when comparing the 1st and 2nd cycle CV curves of highSAC and lowSAC. Since the bulk of SEI formation occurs in the first cycle, the area enclosed by the CV curve during the 2nd cycle is expected to drop as there will be a less significant formation of the passivation layer. When comparing the enclosed areas, the highSAC showed a 26% decrease of the enclosed area from the first to the second cycle, while the lowSAC only showed a 6% decrease (Figure S5 & Table S4). Moreover, there is no oxidation peak in the anodic scan associated to the sodium-metal oxidation with the highSAC, which indicates that, besides the SEI formation, even the deposited sodium metal or ions in the nanopores of highSAC are not electrochemically removable. This is partially responsible to the low capacity of high surface materials. In summary, both the EIS and CV measurements support that more SEI was formed with the high surface area material. Similar phenomenon has been observed on higher-surface-area carbon materials in Li-ion batteries.[7] 14 Figure 7. Natural logarithm (ln) transformed models comparing irreversible capacity percentage of the first cycle versus absolute value of ln pore volume and ln surface area. The best and worst results for the same materials were ploted. It is also well known that SEI formation causes irreversible capacity during the first cycle and is proportional to the measurable surface area of carbon materials.[42,43] To confirm this, we plotted the percentage of irreversible capacity over the discharge capacity in the first cycle vs. the ln transformed pore volume and surface area for all the samples (Figure 7). A linear regression analysis yields reasonable R2 values. Both corresponding p-values, obtained with 30 DF, are below 7∙10-9, thereby implying that there is strong evidence linking irreversible capacity with DFT Pore Volume and Surface Area (Figure S6). We further investigated the electrochemical properties of the two materials. The charge/discharge profiles differ greatly between lowSAC and highSAC. The former displays two distinct features during desodiation. The first is a relatively flat plateau that runs from 0.05 to 0.2 V. This is followed by a sloping curve to 2.0 V. The flat plateau of the desodiation curve can be attributed to the de-insertion of the sodium ions from voids between graphite nanodomains. The sloping curve stems from the sodium-ion de-intercalation out of graphite 15 nanocrystals. The latter eschews these characteristics. Instead it exhibits a capacitor-like behavior where the voltage rises almost linearly from 0.01 V to 2.0 V during the same desodiation process (Figure 8a, b).[9] (b) (a) Figure 8. a) Galvanostatic charge-discharge profiles of lowSAC, carbon pyrolysed at 1100 ºC, unactivated, b) charge-discharge profiles of highSAC, carbon pyrolysed at 1000 ºC and activated at 900 ºC for 10 hrs. The lower reversible capacity for high surface area materials is clearly associated with the loss of capacity obtained below ~ 0.2 V. This portion of capacity can be attributed to the de-insertion of Na+ ions from voids inside the closed hard carbon structure according to the hypothesis by Dahn et al. [6,9] . With higher porosity generated, such closed hard carbon structure has been destroyed, which is responsible for the observed loss of capacity at low voltage, e.g. below 0.2 V. The capacity obtained at higher than 0.2 V may be from either the de-intercalation of ions from graphitic structures or electrical double layer capacitance. [9,44] In order to quantify the loss of capacity at low voltages, a ratio of the reversible capacity under 0.2 V over the total reversible capacity was calculated (Table S5). 16 When comparing these ratios to the respective measurable specific surface areas, we discovered that low surface area materials typically obtain around 50% of their reversible capacity below 0.2 V. On the other hand, higher surface area materials, notably the 900 °C5hrs and 900 °C-10hrs samples, obtain only ~10% of their reversible capacity under 0.2 V. The aforementioned ratio vs. surface area was plotted and fitted with a linear regression model that yields an R2 value of 0.831 and a p-value of 4.2∙10-13 (Figure S7). When increasing open porosity of hard carbon, the volume of the closed hard carbon structure conducive for Na+ storage is certainly diminished. This is why the capacity is inversely proportional to the specific pore volume/surface area. Cyclic voltammetry (CV) was conducted at a scanning rate of 0.2 mV s-1 for lowSAC. During the initial cathodic scan, there is a broad reduction plateau between 0.2 to 0.6 V, indicative of SEI formation. The CV curves have sharp reduction and oxidation peaks between 0.01 V to 0.2 V. The reduction peak is attributed to insertion of Na+ into the voids in hard carbon, while the oxidation peak signifies their removal (Figure 9a).[45] These results agree well with the charge/discharge profiles, as the charge and discharge curve plateau when the voltage is below 0.2 V. CV was also conducted on the highSAC, but it was found that the oxidation peak associated to sodium-metal oxiation in the anodic scan occurring between 0.01 and 0.2 V had mostly vanished (Figure S5). Instead, the CV curve for the high surface area material begins to adopt the characteristics of a capacitor. This corroborates what was seen in the charge/discharge curves as well as the observation that increased surface area and porosity lead to a decrease of Na+ ion storage features, which leads to the loss of reversible capacity obtained at voltages below 0.2 V. 17 Figure 9. Electrochemical characterizations of C-1100. a) CV curves for the first three cycles at a scanning rate of 0.2 mV s-1, b) galvanostatic cycling at different current rates , c) long term galvanostatic cycling at a high rate of 300 mA g-1. We also investigated the cycling performance of the C-1100 with different current rates (Figure 9b). Decreases in specific capacity are observed at higher currents, though this is expected due to the kinetic limitation of hard carbon. After 100 cycles, the carbon material showed almost no loss in capacity at 40 mA g-1 when compared to the capacities of the initial 18 cycles. A long term cycling at 300 mA g-1 was also conducted, and after 500 cycles, 70% of the initial capacity remained (Figure 9c). 4. Conclusion We demonstrate that there is an inherent relationship between the porosity and reversible capacity: increases of measurable porosity are very strongly associated with lower reversible capacities. This insight may be applied to other bio-mass converted hard carbons as anodes in SIBs, as it reveals that superior Na+ ion storage in such batteries is dependent on the absence of pores detectable through N2 sorption. Using a low measurable surface area/porosity hard carbon we are able to achieve a reversible capacity of 335 mAh g-1 at a current rate of 40 mA g-1 along with a relatively stable long term cycling (500 cycles) at 300 mA g-1. In our laboratories, there are ongoing efforts in search of yet lower pore volumes aimed for further increasing reversible capacity while lowering irreversible capacity. 5. Acknowledgements X. J. gratefully acknowledges the financial support from Advanced Research Projects Agency-Energy (ARPA-E), Department of Energy of the United States, Award Number: DEAR0000297TDD. We thank Dr. Jong-Jan Lee, Dr. Yuhao Lu, Dr. Sean Vail and Dr. Long Wang from Sharp Labs America for their suggestions and assistance. The authors would like to thank Dr. Peter Eschbach and Ms. Teresa Sawyer for the SEM measurements in OSU EM Facility funded by National Science Foundation, the Murdock Charitable Trust and the Oregon Nanoscience and Microtechnology Institute. We appreciate Professor Chih-Hung Chang and Mr. Changqing Pan for Raman spectra studies. Lastly, we are grateful to Professor Michael M. Lerner for his advice. 19 References [1] L. Wang, Y. Lu, J. Liu, M. Xu, J. Cheng, D. Zhang et al. A Superior Low-Cost Cathode for a Na-Ion Battery. Angew. Chem Int. Ed. 2013; 52: 1964. [2] B. Dunn, H. Kamath, J. M. Tarascon. Electrical energy storage for the grid: a battery of choices. Science 2011; 334: 928. [3] M. D. Slater, D. Kim, E. Lee, C. S. Johnson. Sodium-Ion Batteries. Adv. Funct. Mater. 2011; 23: 947-949. [4] I. A. Udod, H. B. Orman, V. K. Genchel. The sodium-graphite system under high- pressure conditions: The comparison with the lithium-graphite system. Carbon 1994; 32: 101105. [5] V. V. Avdeev, V. A. Nalimova, K. N. Semenenko. Sodium-graphite system at high pressures. Synthetic Metals 1990; 38: 363-369. [6] D. A. Stevens, J.R. Dahn. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001; 148: A805. [7] S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, et al. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011; 21: 2859-2863. [8] K. Gotoh, T. Ishikawa, S. Shimadzu, N. Yabuuchi, S. Komaba, K. Takeda, et al. NMR study for electrochemically inserted Na in hard carbon electrode of sodium ion battery J. Power Sources 2013; 225: 137. [9] D. A. Stevens, J. R. Dahn. High Capacity Anode Materials for Rechargeable Sodium‐ Ion Batteries. J Electrochem. Soc. 2000; 147: 1271. [10] X. Xia, M. N. Obravac, J.R. Dahn. Comparison of the Reactivity of NaxC6 and LixC6 with Non-Aqueous Solvents and Electrolytes. Electrochem. Solid-State Lett. 2011; 14: A130. 20 [11] Y. Kim, Y. Park, A. Choi, N. S. Choi, J. Kim, J. Lee, et al. An amorphous red phosphorus/carbon composite as a promising anode material for sodium ion batteries. Adv. Mater. 2013; 25: 3045. [12] J. F. Qian, X. Y. Wu, Y. L. Cao, X. P. Ai, H. X. Yang. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angw. Chem. Int. Ed. 2013; 52: 4633. [13] Y. Park, D. Shin, S. H. Woo, N. S. Choi, K. H. Shin, S. M. Oh, et al. Sodium terephthalate as an organic anode material for sodium ion batteries. Adv. Mater. 2012; 24: 3562. [14] H. Xiong, M. D. Slater, M. Balasubramanian, C. S. Johnson, T. Rajh. Amorphous TiO2 Nanotube Anode for Rechargeable Sodium Ion Batteries. J. Phys. Chem. Lett. 2011; 2: 245. [15] R. Alcántra, M. Jaraba, P. Lavela, J. T. Tirado. NiCo2O4 Spinel: First Report on a Transition Metal Oxide for the Negative Electrode of Sodium-Ion Batteries. Chem. Mater. 2002; 14: 287. [16] Z. H. Bi, M. P Paranthaman, P. A. Menchhofer, R. R. Dehoff, C. A. Bridges, M. F. Chi, et al. Self-organized amorphous TiO2 nanotube arrays on porous Ti foam for rechargeable lithium and sodium ion batteries. J. Power Sources 2013; 222: 461. [17] X. Li, M. M. Hasan, A. L. Hector, J. R. Owen. Performance of nanocrystalline Ni3N as a negative electrode for sodium-ion batteries. J. Mater. Chem. A. 2013; 1: 6441. [18] P. Senguttuvan, G. Rousse, V. Seznec, J. M. Tarascon, M. R. Palacin. Na2Ti3O7: Lowest Voltage Ever Reported Oxide Insertion Electrode for Sodium Ion Batteries. Chem. Mater. 2011; 23: 4109. [19] P. Senguttuvan, G. Rousse, M. E. Arroyo y Dompablo, H. Vezin, J. M. Tarascon, M. R. Palacin. Low-potential sodium insertion in a NASICON-type structure through the Ti(III)/Ti(II) redox couple. J. Am. Chem. Soc. 2013; 135: 3897. 21 [20] Y. Park, D. S. Shin, S. H. Woo, N. S. Choi, K. H. Shin, S. M. Oh, et al. Sodium terephthalate as an organic anode material for sodium ion batteries. Adv. Mater. 2012; 24: 3562. [21] L. Zhao, J. M. Zhao, Y. S. Hu, H. Li, Z. B. Zhou, M. Armand, et al. Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost RoomTemperature Sodium-Ion Battery. Adv. Energy Mater. 2012; 2: 962. [22] S. I. Park, I. Gocheva, S. Okada, J. I. Yamaki. Electrochemical Properties of NaTi2(PO4)3 Anodes for Rechargeable Aqueous Sodium-Ion Batteries. J. Electrochem. Soc. 2011; 158: A1067. [23] Q. Sun, Q. Q. Ren, H. Li, Z. W. Fu. High capacity Sb2O4 thin film electrodes for rechargeable sodium battery. Electrochem. Comm. 2011; 13: 1462. [24] Y. Liu, Y. Xu, Y. Zhu, J. Culver, C.A. Lundgren, K. Xu, et al. Tin-Coated Viral Nanoforests as Sodium-Ion Battery Anodes. ACS Nano 2013; 7: 3627. [25] A. Darwiche, C. Marino, M. T. Sougratti, B. Fraise, L. Stievano, L. Monconduit. Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: an unexpected electrochemical mechanism. J. Am. Chem. Soc. 2012; 134: 20805. [26] J.F. Qian, Y. Chen, L. Wu, Y. L. Cao, X. P. Ai, H. X. Yang. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Comm. 2012; 48: 7070. [27] Y. H. Xu, Y. J. Zhu, Y. J. Liu, C. S. Wang. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013; 3: 128. [28] J. W. Wang, X. H. Liu, S. X. Mao, J. Y. Huang. Microstructural evolution of tin nanoparticles during in situ sodium insertion and extraction. Nano Lett. 2012; 12: 5897. 22 [29] M. K. Datta, R. Epur, P. Saha, K. Kadakia, S. K. Park, P. N. Kuma. Tin and graphite based nanocomposites: Potential anode for sodium ion batteries. J. Power Sources 2013; 225: 316. [30] V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González, T. Rojo. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy and Environ. Sci. 2012; 5: 5884-5901. [31] R. Alcántara, J. M. Jiménez-Mateos, P. Lavela, J. L. Tirado. Carbon black: a promising electrode material for sodium-ion batteries. Electrochemistry Comm. 2001; 3: 639640. [32] R. Alcántara, P. Lavela, G. F. Ortiz, J. L. Tirado. Carbon Microspheres Obtained from Resorcinol-Formaldehyde as High-Capacity Electrodes for Sodium-Ion Batteries. Electrochem. Solid-State Lett. 2005; 8: A222-A225. [33] S. Wenzel, T. Hara, J. Janek, P. Adelhelm. Room-temperature sodium-ion batteries: improving the rate capability of carbon anode materials by templating strategies. Energy Environ. Sci. 2011; 4: 3342. [34] Z.H. Wang, L. Qie, X. Yuan, W. X. Zhang, X. L. Lu, Y. H. Huang. Functionalized N- doped interconnected carbon nanofibers as an anode material for sodium-ion storage with excellent performance. Carbon 2013; 55: 328. [35] H. G. Wang, Z. Wu, F. L. Meng, D. L. Ma, X. L. Huang, L. M. Wang, et al. Nitrogen- doped porous carbon nanosheets as low-cost, high-performance anode material for sodiumion batteries. ChemSusChem 2013; 6: 56. [36] D. Su, H. J. Ahn, G. Wang. SnO2@graphene nanocomposites as anode materials for Na-ion batteries with superior electrochemical performance. Chem. Comm. 2013; 49: 3131. [37] K. Tang, L. Fu, R. J. White, L. Yu. Hollow Carbon Nanospheres with Superior Rate Capability for Sodium-Based Batteries. Adv. Energy Mater. 2012; 2: 873. 23 [38] Y. L. Cao, L. F. Xiao, M. L. Sushko, W. Wang, B. Schwenzer, J. Xiao, et al. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012; 12: 3783. [39] A. Ponrouch, A. R. Goni, M. Rosa Palacin. Towards high energy density sodium ion batteries through electrolyte optimization. Electrochem. Comm. 2013; 25: 85. [40] Y. Chuan-Lin, J. Cho, G. A. Tompsett, P. R. Westmoreland, G. W. Huber. Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C 2009; 113: 20097. [41] P. Ravikovitch, A. Vishnyakov, R. Russo, A. V. Neimark. Unified Approach to Pore Size Characterization of Microporous Carbonaceous Materials from N2, Ar, and CO2 Adsorption Isotherms. Langmuir 2000; 16: 2316. [42] R. Fong, U. Sacken, J. R. Dahn. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc 1990; 7: 2010 [43] M. Winter, P. Novak, A. Monnier. Graphite for Lithium-Ion Celles: The Correlation of the First-Cycle Charge Loss with the BET Surface Area. J. Electrochem. Soc. 1998; 2:428 [44] Y. Shao, J. Xiao, W. Wang, M. Engelhard, J. Liu et al. Surface-Driven Sodium Ion Energy Storage in Nanocellular Carbon Foams. Nano Letters 2013; 13: 3909-3910. [45] M. K. Datta, R. Epur, P. Saha, K. Kadakia, S. K. Park and P. N. Kuma. Tin and graphite based nanocomposites: Potential anode for sodium ion batteries. J. Power Sources 2013; 225: 316. 24 Supporting Information Predicting Capacity of Hard Carbon Anodes in Sodium-Ion Batteries Using Porosity Measurements Clement Bommier1, Wei Luo1, Wen-Yang Gao3, Alex Greaney2, Shengqian Ma3 and Xiulei Ji1 1. Department of Chemistry, Oregon State University, Corvallis, Oregon, 97331, USA 2. Department of Mechanical, Industrial and Materials Engineering, Oregon State University, Corvallis, Oregon, 97331, USA 3. Department of Chemistry, University of South Florida, Tampa, Florida, 33620, USA Corresponding author: Tel: +1 541-737-6798, E-mail: david.ji@oregonstate.edu 25 + Potential (V vs. Na/Na ) 2.0 1.5 1.0 0.5 0.0 0 1 Specific Capacity (mAh g ) 300 250 (b) Super P (a) 30 60 100 200 300 400 -1 Specific Capacity (mAh g ) 120 300 600 1200 600 300 120 60 30 -1 mA g 200 150 100 50 0 0 5 10 15 20 25 30 35 40 Cycle Number 45 50 55 60 Figure S1. Electrochemical performance of Super-P, a) Galvanostatic first cycle charge/discharge profiles, b) cycling performance at different rates. 26 Figure S2. Raman Spectra shifts for a) 900 ºC CO2 activation series b) 700 ºC -10hrs and 800 ºC-10hrs CO2 activation c) Different Pyrolysis temperatures. The untreated label refers to the sucrose sample pyrolysed at 1100 ºC. The peak at 1350 cm-1 is from the disordered carbon, while the peak at 1580 cm-1 is from the graphitic carbon. 27 (b) (a) Residual standard error: 24.03 on 30 degrees of freedom Multiple R-squared: 0.8625, Adjusted R-squared: 0.8579 F-statistic: 188.2 on 1 and 30 DF p-value: 1.842e-14 Residual standard error: 29.98 on 30 degrees of freedom Multiple R-squared:0.7859 Adjusted R-squared: 0.7788 F-statistic: 110.1 on 1 and 30 DF p-value: 1.468e-11 (d) (c) Residual standard error: 27.54 on 30 degrees of freedom Multiple R-squared: 0.8194 Adjusted R-squared:0.8133 F-statistic: 136.1 on 1 and 30 DF p-value: 1.127e-12 Residual standard error: 26.56 on 30 degrees of freedom Multiple R-squared: 0.8319 Adjusted R-squared: 0.8263 F-statistic: 148.5 on 1 and 30 DF p-value: 3.792e-13 Figure S3. Linear regression models of a) Reversible capacity vs. measurable DFT pore volume b) Reversible capacity vs. ln transformed DFT pore volume c) Reversible capacity vs. measurable surface area d) Reversible capacity vs. ln transformed surface area. 28 Residual standard error: 21.48 on 30 degrees of freedom Multiple R-squared: 0.8901, Adjusted R-squared: 0.8865 F-statistic: 243 on 1 and 30 DF, p-value: 6.268e-16 Figure S4. Linear regression model of reversible capacity vs. logit transform of percentage of the unexposed carbon atoms. The logit transform is obtained through 29 (a) (b) Figure S5. CV curves of (a) lowSAC, (b) highSAC. Scanning rate: 0.2 mV/s 30 (a) (b) Residual standard error: 0.1119 on 30 degrees of freedom Multiple R-squared: 0.681, Adjusted R-squared: 0.6703 F-statistic: 64.03 on 1 and 30 DF p-value: 6.233e-09 Residual standard error: 0.108 on 30 degrees of freedom Multiple R-squared: 0.7031, Adjusted R-squared: 0.6932 F-statistic: 71.05 on 1 and 30 DF p-value: 2.087e-09 Figure S6. Linear regression models of a) Irreversible capacity vs. ln transformed DFT pore volume b) Irreversible capacity vs. ln transformed surface area. 31 2 Incremental Surface Area (m ) 80 60 900 C-1h 40 20 0 0 50 100 Pore Size (Å) 150 2 400 Incremental Surface Area (m ) 2 Incremental Surface Area (m ) 2 Incremental Surface Area (m ) (a) 300 900 C-5h 200 100 0 0 50 100 Pore Size (Å) 150 300 250 200 900 C-2h 150 100 50 0 0 50 100 Pore Size (Å) 150 600 500 400 900 C-10h 300 200 100 0 0 50 100 Pore Size (Å) 150 2 Incremental Surface Area (m ) 800 C-1h 20 15 10 5 0 0 50 100 Pore Size (Å) 150 2 2 25 Incremental Surface Area (m ) 10 2 Incremental Surface Area (m ) 30 Incremental Surface Area (m ) (b) 8 800 C-5h 6 4 2 0 0 50 100 Pore Size (Å) 150 32 40 35 800 C-2h 30 25 20 15 10 5 0 0 50 100 Pore Size (Å) 150 40 35 30 800 C-10h 25 20 15 10 5 0 0 50 100 Pore Size (Å) 150 2 1 0 50 20 700 C-5h 15 10 5 0 5 0 0 50 100 Pore Size (Å) 150 1100 C 0.4 0.3 0.2 0.1 0.0 50 100 Pore Size (Å) 150 1.2 1.0 900 C 0.8 0.6 0.4 0.2 0.0 -0.2 0 50 100 Pore Size (Å) 150 150 8 700 C-10h 6 4 2 0 0 0.6 50 2 0 50 100 Pore Size (Å) 10 2 Incremental Surface Area (m ) Incremental Surface Area (m ) 1.4 2 10 2 25 700 C-2h 15 150 30 0.5 2 100 20 Incremental Surface Area (m ) 0 0 (d ) Incremental Surface Area (m ) 700 C-1h 2 -1 25 Incremental Surface Area (m ) 2 Incremental Surface Area (m ) 2 Incremental Surface Area (m ) 3 Incremental Surface Area (m ) (c) 50 100 Pore Size (Å) 150 0.5 0.4 1000 C 0.3 0.2 0.1 0.0 0 50 100 Pore Size (Å) 150 40 30 800 C 20 10 0 0 50 100 Pore Size (Å) 150 Figure S6. Pore size distribution (DFT model) calculated using 77 K N2 sorption data for a) samples activated at 900 °C b) samples activated at 800 °C c) samples activated at 700 °C d) Samples prepared at differing pyrolysis temperatures. Before CO2 activation, the carbon sample was originally pyrolysed at 1000°C. 33 Residual standard error: 0.05634 on 30 degrees of freedom Multiple R-squared:0.8307 Adjusted R-squared: 0.825 F-statistic: 147.2 on 1 and 30 DF p-value: 4.243e-13 Figure S7. The ratio of capacity obtained below 0.2 V over the total reversible capacity as a function of measurable specific surface area. 34 Table S1. The table represents the calculated Id/Ig ratios obtained. Active Material 900 ºC -1hr 900 ºC -2hrs 900 ºC -5hrs 900 ºC -10hrs Id/Ig 1.028 1.021 1.039 1.042 Active Material 700 ºC -10hrs 800 ºC -10hrs Id/Ig 0.974 1.003 Active Material 800 ºC 900 ºC 1000 ºC (untreated) 1100 ºC Id/Ig 1.009 1.018 1.031 1.035 Table S2. Elemental analysis of hard carbons obtained at different pyrolysis temperatures. Pyrolysis Temperature 800 °C 900 °C 1000 °C 1100 °C Carbon Mass % Hydrogen Mass % Oxygen Mass % 95.49 93.79 96.20 97.44 0.60 0.39 0.21 0.31 3.91 5.82 3.59 2.25 35 Table S3. Respective percentages of exposed and exposed carbon atoms. The logit transform is obtained through the equation . This was done to spread out the clustered percentage values that were all above 90%. Material 900 ºC-1hr 900 ºC-2hrs 900 ºC-5hrs 900 ºC-10hrs 800 ºC-1hr 800 ºC-2hrs 800 ºC-5hrs 800 ºC-10hrs 700 ºC-1hr 700 ºC-2hrs 700 ºC-5hrs 700 ºC-10hrs 800 ºC 900 ºC 1000 ºC 1100 ºC % Exposed Carbon atoms 14.2% 22.0% 53.9% 91.3% 6.6% 10.0% 10.1% 16.6% 9.8% 8.2% 9.2% 12.7% 17.2% 6.0% 3.8% 1.6% % Unexposed Carbon atoms 85.8% 78.0% 46.1% 8.7% 93.4% 90.0% 89.9% 83.4% 90.2% 91.8% 90.8% 87.3% 82.8% 94.0% 96.2% 98.4% logit(unexposed Carbon atoms) 1.80 1.26 -0.16 -2.35 2.64 2.20 2.19 1.61 2.21 2.42 2.29 1.93 1.57 2.75 3.23 4.12 Table S4. Calculated areas enclosed in the 1st and 2nd cycle CV curves for low surface area carbon, lowSAC, and high surface area carbon, highSAC. Material lowSAC highSAC Area of 1st Cycle 0.373 0.249 36 Area of 2nd Cycle 0.351 0.184 Percentage drop 6.1% 26.1% Table S5. Ratio of capacities obtained under 0.2 V over the total reversible capacity obtained. The ratios are expressed in % values. The best and worst results for the same materials are shown here. Reversible Capacity (mAh g-1) Capacity Under 0.2 V (mAh g1 ) Capacity % under 0.2 V 900 ºC-1hr 239.3 122.6 51.2% 900 ºC-1hr 214.9 114.8 900 ºC-2hrs 208.0 900 ºC-2hrs 900 ºC-5hrs Material Reversible Capacity (mAh g-1) Capacity Under 0.2 V (mAh g-1) Capacity % under 0.2 V 800 ºC-1hr 257.4 141.7 55.0% 53.4% 800 ºC-1hr 229.6 124.5 54.2% 101.6 48.8% 800 ºC-2hrs 240.6 140.3 58.3% 186.5 86.2 46.2% 800 ºC-2hrs 236.6 132.6 56.0% 107.0 14.3 13.3% 800 ºC-5hrs 218.5 103.9 47.6% 800 ºC-5hrs 221.7 104.1 46.9% Material 900 ºC-5hrs 120.7 20.5 17.0% 900 ºC-10hrs 49.3 6.7 13.6% 800 ºC-10hrs 268.3 158.9 59.2% 900 ºC-10hrs 43.6 4.5 10.4% 800 ºC-10hrs 228.9 126.2 55.1% Material Reversible Capacity (mAh g-1) Capacity Under 0.2 V (mAh g1 ) Capacity % under 0.2 V Material Reversible Capacity (mAh g-1) Capacity Under 0.2 V (mAh g-1) Capacity % under 0.2 V 700 ºC-1hr 232.5 122.7 52.8% 800 ºC 199.8 80.0 40.0% 700 ºC-1hr 225.3 117.1 52.0% 800 ºC 221.4 108.7 49.1% 700 ºC-2hrs 205.8 108.7 52.8% 900 ºC 260.9 142.2 54.5% 700 ºC-2hrs 192.7 96.9 50.3% 900 ºC 248.2 108.5 43.7% 700 ºC-5hrs 263.5 134.3 51.0% 1000 ºC 282.8 149.6 52.9% 1000 ºC 290.4 156.3 53.8% 700 ºC-5hrs 255.1 128.0 50.2% 700 ºC-10hrs 249.8 130.8 52.3% 1100 ºC 335.0 160.8 48.0% 700 ºC-10hrs 241.5 129.0 53.4% 1100 ºC 299.0 173.8 58.1% 37