A microwave spectroscopic and quantum chemical study of propa-1,2-dienyl selenocyanate (H
advertisement

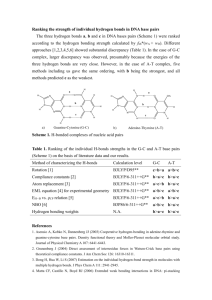
PAPER www.rsc.org/pccp | Physical Chemistry Chemical Physics A microwave spectroscopic and quantum chemical study of propa-1,2-dienyl selenocyanate (H2QCQCHSeCRN) and cyclopropyl selenocyanate (C3H5SeCRN)w Rajmund Mokso,a Harald Møllendal*a and Jean-Claude Guilleminb Received 28th February 2008, Accepted 16th April 2008 First published as an Advance Article on the web 2nd June 2008 DOI: 10.1039/b803562h The microwave spectra of propa-1,2-dienyl selenocyanate, H2CQCQCHSeCRN, and cyclopropyl selenocyanate, C3H5SeCRN, are reported. The spectra of the ground and two vibrationally excited states of the 80Se isotopologue and the spectrum of the ground state of the 78 Se isotopologue were assigned for one rotameric form of H2CQCQCHSeCRN. This conformer is characterized by a C–C–Se–C dihedral angle of 129(5)1 from synperiplanar (01) and is shown to be the global minimum of H2CQCQCHSeCRN. The spectra of the ground and of three vibrationally excited states of the 80Se isotopologue, as well as of the ground state of the 78 Se isotopologue of one rotamer of C3H5SeCRN were assigned. This conformer has a H–C–Se–C dihedral angle of 80(4)1 from synperiplanar and is at least 3 kJ mol 1 more stable than any other form of the molecule. The microwave study has been augmented by quantum chemical calculations at the B3LYP/6-311++G(3df,3pd) and MP2/6-311++G(3df,3pd) levels of theory. 1. Introduction Very few studies of the structural and conformational properties of organic selenocyanates (X–Se–CRN) in the gaseous state have been performed. In one such study, the structure, dipole moment and barrier to internal rotation of the methyl group of methyl selenocyanate (CH3SeCN) were investigated by Sakaizumi et al.1,2 employing microwave (MW) spectroscopy. The conformational properties of ethyl selenocyanate (CH3CH2SeCN) was the theme of another MW study.3 The spectrum of only one rotamer, which has a synclinal (obsolete ‘‘gauche’’) conformation for the C–C–Se–C link of atoms, was identified in this investigation, whereas the spectrum of the antiperiplanar (‘‘trans’’) form was not observable. Very recently, the MW spectrum of etheneselenocyanate, H2CQCHSeCN, was reported revealing the existence of two rotameric forms of this compound. The dihedral angle formed by the C–C–Se–C chain of atoms is 01 in the synperiplanar (‘‘cis’’) conformer and takes the unusual value of 166(3)1 in the second anticlinal (‘‘skew’’) rotamer of this molecule.4 The synperiplanar rotamer of this compound is more stable by 4.5(4) kJ mol 1.4 Recently, several new selenocyanates have been synthesized5–8 and this has made it possible to investigate their physical properties. The syntheses and photoelectron spectra of several allenyl selenocyanates, including the two title compounds H2CQCQCHSeCN7 and cyclopropylselenocyanate (C3H5SeCN),8 have already been reported together with quantum chemical calculations.7,8 In H2CQCQCHSeCN, the selenocyanate group is attached to an allenyl group, whereas the selenocyanate group is attached to a cyclopropyl ring in C3H5SeCN. Rotation about the C––Se bonds may produce rotational isomerism in these two cases and interactions between the selenocyanate group and the allenyl, or between the cyclopropyl group and the selenocyanate group, will determine the conformational properties of these two molecules. The methods we have used in this study are MW spectroscopy and high-level quantum chemical calculations. MW spectroscopy was chosen because of its extremely high accuracy and resolution, making this method especially suitable for conformational studies. The spectroscopic work has been augmented by high-level quantum chemical calculations, which were conducted with the purpose of obtaining information for the use in assigning the MW spectra and investigating properties on the potential-energy hypersurface. 2. Experimental a Centre for Theoretical and Computational Chemistry (CTCC), Department of Chemistry, University of Oslo, P. O. Box 1033 Blindern, NO-0315 Oslo, Norway. E-mail: harald.mollendal@kjemi.uio.no; Fax: +47 2285 5441; Tel: +47 2285 5674 b Sciences Chimiques de Rennes, École Nationale Supe´rieure de Chimie de Rennes-CNRS, F-35700 Rennes, France w Electronic supplementary information (ESI) available: Syntheses of H2QCQCHSeCRN and (C3H5SeCRN) and their microwave spectra. See DOI: 10.1039/b803562h 4138 | Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 Synthesis of selenocyanic acid propa-1,2-diene ester and selenocyanic acid cyclopropane ester The synthesis of both compounds have been reported recently.7,8 The three-step syntheses are described in Schemes 1 and 2, respectively. A detailed experimental procedure is given in the electronic supplementary information (ESI) for the convenience of the reader.w This journal is c the Owner Societies 2008 Scheme 1 . Fig. 1 The synperiplanar (sp) and anticlinal (ac) rotamers of H2CQCQCHSeCN. Atom numbering is shown on sp. The microwave spectrum of ac was assigned. Scheme 2 . Microwave experiment The MW spectrum was recorded in the 48–80 GHz spectral region in the case of H2CQCQCHSeCN and in the 38–80 GHz range for C3H5SeCN, using the Stark-modulated spectrometer of the University of Oslo. Details of the construction and operation of this spectrometer, which has a 2 m HewlettPackard Stark cell, have been given elsewhere.9,10 The spectrum of H2CQCQCHSeCN was taken with the cell cooled to roughly 0 1C, whereas C3H5SeCN was recorded at about 10 1C. A reduction of the temperature from room temperature was done in order to enhance the intensity of the spectral lines. Lower temperatures, which would have increased the intensity of the spectra even more, could not be achieved, owing to insufficient vapour pressures of both H2CQCQCHSeCN and C3H5SeCN. The spectral lines of these two compounds were found to be broad and were measured with an estimated accuracy of E0.10 MHz. Radio-frequency microwave double resonance experiments (RFMWDR), similar to those performed by Wodarczyk and Wilson,11 were also conducted to assign unambiguously particular transitions. function has two minima at about 0 and 1301 of the C1–C2–Se7–C8 dihedral angle, and maxima at about 50 and 1801. Separate calculations of the structures, energies, dipole moments, vibrational frequencies, Watson’s A-reduction centrifugal distortion constants16 and vibration–rotation constants (the a’s)17 were then undertaken for the two conformers. The starting values of the C1–C2–Se7–C8 dihedral angles were chosen to be close to 0 and 1301, respectively. Full geometry optimizations with no symmetry restrictions were made employing the default convergence criteria of Gaussian 03. The C1–C2–Se7–C8 dihedral angle was found to be exactly 01 (synperiplanar) for one of the conformers, denoted sp, and 130.91 (anticlinal) for the other rotamer, which is henceforth called ac. Only positive values were found for the vibrational frequencies of each of these conformers. The B3LYP structures found in these calculations are listed in Table 1. The electronic energy difference between the two rotamers was predicted to be 3.34 kJ mol 1, with ac as the more stable Table 1 B3LYP and MP2 geometriesa.b of the sp and ac conformers of H2CQCQCHSeCN 3. Results Method: B3LYP Quantum-chemical methods Conformer: sp The present ab initio and density functional theory (DFT) calculations were performed employing the Gaussian 03 suite of programs,12 running on the 64 processor HP ‘‘superdome’’ computer in Oslo. Electron correlation was taken into consideration in the ab initio calculations using Møller–Plesset second-order perturbation calculations (MP2).13 Becke’s three-parameter hybrid functional14 employing the Lee, Yang and Parr correlation functional15 (B3LYP) was employed in the DFT calculations. The 6-311++G(3df,3pd) basis set was employed because this basis set has been optimized for selenium. Bond length/pm C1–C2 C1–C4 C2–H3 C2–Se7 C4–H5 C4–H6 Se7–C8 C8–N9 Angle/1 C1–C2–H3 C1–C2–Se7 C1–C4–H5 C1–C4–H6 C2–Se7–C8 Se7–C8–N9 C2–C1–C4 Dihedral angle/1 C1–C2–Se7–C8 H3–C2–Se7–C8 H3–C2–C4–H5 H3–C2–C4–H6 Se7–C2–C4–H5 Se7–C2–C4–H6 C4–C2–C3–Se7 C1–Se7–C8–N9 Calculations for H2CQCQCHSeCN A model of this compound with atom numbering is shown in Fig. 1. Rotation about the C2–Se7 bond may produce rotational isomerism. B3LYP calculations were performed in an attempt to predict which rotameric forms are minima (‘‘stable’’) of the potential-energy hypersurface. Calculations of energies were performed for the 0 to 1801 interval in steps of 101 of the C1–C2–Se7–C8 dihedral angle, employing the scan option of the Gaussian 03 program, allowing all remaining structural parameters to vary freely. The resulting potential This journal is c the Owner Societies 2008 a MP2 ac sp ac 129.3 130.0 108.3 194.4 108.3 108.3 183.8 115.5 129.6 130.0 108.1 194.0 108.3 108.3 184.7 115.5 130.2 130.9 108.2 192.3 108.2 108.2 182.8 117.6 130.6 130.8 108.2 191.6 108.2 108.2 183.8 117.6 123.9 126.1 121.3 121.3 98.9 177.5 178.9 124.1 120.1 121.4 121.0 97.4 177.8 179.6 123.6 124.1 120.8 120.8 96.1 179.5 177.3 123.2 119.5 120.8 120.5 95.9 178.5 179.3 0.0 180.0 90.4 90.4 89.6 89.6 1.8 179.7 130.9 53.7 89.9 90.0 84.9 95.2 20.6 160.5 0.0 180.0 90.6 –90.6 –89.4 89.4 0.3 179.4 126.0 58.7 89.8 90.3 85.2 94.7 1.5 156.8 Basis set: 6-311++G(3df,3pd). b Atom numbering is given in Fig. 1. Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 | 4139 Table 2 B3LYPa and MP2a parameters of spectroscopic interest of the sp and ac conformers of H2CQCQCH80SeCN Method: B3LYP Conformer: sp Experimentalb MP2 ac sp ac Rotational constant/MHz A 3347.3 5624.7 3461.4 5213.0 B 1879.1 1300.2 1943.7 1346.8 C 1213.5 1114.3 1255.5 1137.5 Quartic centrifugal distortion constantc/kHz 0.505 0.592 0.436 0.820 DJ 4.51 13.5 4.82 14.8 DJK 3.96 190 2.46 169 DK dJ 0.172 0.157 0.134 0.249 2.95 0.993 2.80 0.291 dK Dipole momente/10 30 C m 3.8 14.8 2.1 15.4 ma 11.8 5.6 12.0 6.0 mb f f 0.0 1.3 0.0 1.2 mc 12.4 15.9 12.2 16.6 mtot Energy differenceg/kJ mol 1 DE 3.1 0.0h 0.0i 1.3 5487.4(56) 1325.453(81) 1129.332(89) 0.6970(41) 16.141(21) 190d 0.299(17) 0.993d Basis set: 6-311++G(3df,3pd). b This work. c A-reduction.16 d Fixed at the B3LYP value. e 1 debye = 3.33564 10 30 C m. f For symmetry reasons. g Corrected for zero-point vibrational energy. h Electronic energy: 6853 911.11 kJ mol 1. i Electronic energy: 6848 389.20 kJ mol 1. a form. This energy difference becomes 3.10 kJ mol 1 when the effects of the harmonic zero-point vibrational energies are taken into consideration. This value for the energy difference is listed in Table 2, together with the rotational and quartic centrifugal distortion constants for the most abundant isotopologue H2CQCQCH80SeCN (80Se occuring with 49.8% relative abundance), and principal-axes dipole moment components. The two maxima of the potential function were explored next using the transition-state option of Gaussian 03. The first maximum was found for a value of 49.61 of the C1–C2–Se7–C8 dihedral angle and an electronic energy that is 4.86 kJ mol 1 higher than the energy of ac. The second maximum was found at exactly 1801 (2.27 kJ mol 1 above the energy of ac). Each of these maxima has one imaginary vibrational frequency associated with the torsion about the C2–Se7 bond, which indicates that they are first-order transition states. The full electronic potential function for rotation about the C2–Se7 bond could now be drawn, as shown in Fig. 2. MP2/6-311++G(3df,3pd) calculations of the structure, dipole moment, vibrational frequencies and quartic centrifugal distortion constants were repeated for the sp and ac rotamers, because we wanted to compare results obtained by the MP2 procedure with the counterparts calculated by the B3LYP method and with the relevant experimental findings. Selected MP2 results are included in Tables 1 and 2. The sp form is found to have exact Cs symmetry in both procedures, whereas the MP2 C1–C2–Se7–C8 dihedral angle was predicted to be 126.01 in the ac, about 61 less than found in the B3LYP calculations. The MP2 CQC bond distances are typically 1 pm longer in MP2 than in the B3LYP calculations. The C–H bond lengths are practically the same in both methods. The C2–Se7 distances are over 2 pm longer in the B3LYP than in the MP2 4140 | Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 Fig. 2 B3LYP/6-311++G(3df,3pd) electronic potential function for rotation about the C2–Se7 bond in H2CQCQCHSeCN. The values of the C1–C2–Se7–C8 dihedral angle in degree are given on the abscissa and the relative energies in kJ mol 1 are given on the ordinate. sp has a C1–C2–Se7–C8 dihedral angle of 01. A dihedral angle of 130.91 is predicted for ac. sp is calculated to be 3.34 kJ mol 1 less stable than ac. This potential function has maxima at 49.61 (4.86 kJ mol 1 above the energy of ac), and at 1801 (2.27 kJ mol 1 above the energy of ac). calculations, whereas the B3LYP Se7–C8 bond lengths are longer by about 1 pm. There is also a significant difference in the predicted C8RN9 bond length, which is about 2 pm longer in the MP2 calculations. Bond angles calculated with the two procedures generally agree to within 11, with the exception of the C2–Se7–C8 angle, which is predicted to be 1.5–2.71 larger in the B3LYP calculations. The energy difference corrected for zero-point vibrational effects was predicted to be 1.24 kJ mol 1, with sp as more stable in the MP2 case. This is the opposite energy order of what was found in the B3LYP calculations above. The B3LYP and MP2 results for the energy difference between the two forms therefore vary by 4.34 kJ mol 1, which are not unexpected given the large number of electrons (66) involved in these calculations and the inherent approximations of the two methods. MP2 and B3LYP calculations of the electronic energy difference between sp and ac have been reported previously.7 In this case, the cc-pVTZ wave function18 was used. ac was found to be preferred by 0.76 kJ mol 1 in these B3LYP calculations, whereas the opposite was the case in the MP2 calculations (0.11 kJ mol 1 in favour of sp). These calculations therefore predict a smaller energy difference between the sp and ac than was found in the present calculations. Calculations for C3H5SeCN Rotation about the C1–Se9 bond (atom numbering in Fig. 3) may produce rotational isomerism in this compound. The H4–C1–Se9–C10 dihedral angle is conveniently used to define the rotational isomerism in this case. B3LYP and MP2 calculations analogous to those described above for H2CQCQCHSeCN were performed. This journal is c the Owner Societies 2008 Table 3 MP2 and B3LYP geometriesab of the sc and ap conformers of C3H5SeCN Fig. 3 Model of the synclinal (sc) and antiperiplanar (ap) conformers of cyclopropyl selenocyanate with atom numbering given on sc. The MW spectrum of sc was assigned. This rotamer is at least 3 kJ mol 1 more stable than ap. The B3LYP electronic-energy potential function for rotation about the C1–Se9 bond, which is shown in Fig. 4, has minima at 76.0 synclinal and 180.01 antiperiplanar and maxima at 0 and 146.01 for the H4–C1–Se9–C10 dihedral angle. The rotamers corresponding to these two minima are henceforth denoted sc and ap, respectively. The electronic energy of ap is predicted to be 6.73 kJ mol 1 higher than that of sc. The two ‘‘stable’’ rotamers, ap and sc, are sketched in Fig. 3. The maximum at 01 is 12.49 kJ mol 1 higher in energy than sc, and the maximum at 146.01 is 7.58 kJ mol 1 higher. The energy difference between ap and sc is calculated to be 6.18 kJ mol 1, when contributions from zero-point vibrational effects are taken into consideration. The structures of the two forms are listed in Table 3, and selected parameters of spectroscopic interest are found in Table 4. Method: B3LYP Conformer: sc Bond length/pm C1–C2 C1–C3 C1–H4 C1–Se9 C2–C3 C2–H5 C2–H6 C3–H7 C3–H8 Se9–C10 C10–N11 Angle/1 C2–C1–H4 C2–C1–Se9 C3–C1–H4 C3–C1–Se9 H4–C1–Se9 C1–C2–H5 C1–C2–H6 C3–C2–H5 C3–C2–H6 H5–C2–H6 C1–C3–H7 C1–C3–H8 C2–C3–H7 C2–C3–H8 H7–C3–H8 C1–Se9–C10 Se9–C10–N11 Dihedral angle/1 H4–C1–C2–H5 H4–C1–C2–H6 Se9–C1–C2–H5 Se9–C1–C2–H6 H4–C1–C3–H7 H4–C1–C3–H8 Se9–C1–C3–H7 Se9–C1–C3–H8 C2–C1–Se9–C10 C3–C1–Se9–C10 H4–C1–Se9–C10 H5–C2–C3–H7 H5–C2–C3–H8 H6–C2–C3–H7 H6–C2–C3–H8 C1–Se9–C10–N11 a Fig. 4 B3LYP/6-311++G(3df,3pd) electronic potential function for rotation about the C1–Se9 bond of C3H5SeCN. The values of the H4–C1–Se9–C10 dihedral angle in degree are given on the abscissa and the relative energies in kJ mol 1 are given on the ordinate. sc is calculated to have a H4–C1–Se9–C10 dihedral angle of 76.01, whereas a dihedral angle of exactly 1801 is predicted for ap. sc is calculated to be 6.73 kJ mol 1 more stable than ap. This potential function has maxima at 01 (12.49 kJ mol 1 above the energy of sc), and at 146.01 (7.58 kJ mol 1 above the energy of sc). This journal is c the Owner Societies 2008 MP2 ap sc ap 150.5 149.8 107.9 194.3 150.3 108.1 108.1 108.0 108.1 184.7 115.5 149.3 149.3 108.2 196.2 151.3 108.1 108.1 108.1 108.1 184.0 115.6 150.0 150.8 108.0 191.5 150.2 107.9 107.9 108.0 107.9 183.8 117.6 149.5 149.5 108.2 193.4 151.2 108.0 108.1 108.1 108.0 182.9 117.7 118.2 116.9 118.7 120.9 112.5 117.0 118.3 119.2 117.5 114.5 118.1 116.6 117.8 118.7 114.7 98.0 178.1 118.5 123.9 118.5 123.9 106.1 117.5 118.2 119.2 116.8 114.7 118.2 117.5 116.8 119.2 114.7 97.9 178.9 118.3 119.2 118.0 116.5 114.3 116.3 117.3 118.2 117.7 115.7 117.6 116.6 117.3 118.6 115.6 96.2 179.3 118.2 122.5 118.2 122.5 108.3 117.3 117.5 118.7 116.4 115.7 117.5 117.3 116.4 118.7 115.7 95.2 179.2 1.1 144.4 138.4 4.9 144.4 1.6 2.4 145.3 142.2 72.6 76.0 145.9 0.0 0.2 145.6 177.7 0.8 145.2 137.4 7.1 145.2 0.8 7.1 137.4 37.6 37.6 180.0 144.9 0.0 0.0 144.9 178.7 1.3 144.3 145.6 2.5 144.6 0.9 2.8 140.8 135.3 66.6 81.6 146.5 0.0 0.4 146.9 147.0 0.6 145.7 139.2 5.9 145.7 0.6 5.9 139.2 36.8 36.8 180.0 145.5 0.0 0.0 145.5 0.8 Basis set: 6-311++G(3df,3pd). b Atom numbering is given in Fig. 3. It was found again in the subsequent MP2 calculations that ap and sc are minima on the potential-energy hypersurface, with the results summarized in Tables 3 and 4. The H4–C1–Se9–C10 dihedral angle was predicted to be exact 1801 in ap and 81.61 in sc. The MP2 energy difference corrected for zero-point vibrational effects was calculated to be 4.53 kJ mol 1, with sc as the preferred form relative to ap, compared to 6.18 kJ mol 1 found in the B3LYP calculations. The equilibrium structure of the prototype compound cyclopropane is known.19 The equilibrium bond lengths of C–C and C–H are 151.007(77) and 107.415(98) pm, respectively,19 which are close to their counterparts in Table 3. Comparison of the bond lengths and bond angles (Table 3) of the B3LYP and MP2 structures reveals differences similar Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 | 4141 Table 4 B3LYPa and MP2a parameters of spectroscopic interest of the sc and ap conformers of C3H580SeCN Method: B3LYP Conformer: sc Experimentalb MP2 ap sc ap Rotational constant/MHz A 4341.1 3198.0 4095.4 3140.1 4176.4(19) B 1729.0 2124.4 1846.3 2268.4 1812.965(96) C 1306.1 1421.3 1346.8 1470.8 1338.271(99) Quartic centrifugal distortion constantc/kHz 0.966 0.724 1.31 0.601 1.2605(29) DJ 8.17 1.12 9.59 2.37 9.5769(87) DJK 30.7 0.665 28.4 2.41 30.7d DK dJ 0.407 0.285 0.567 0.229 0.407d dK 0.696 0.274 0.643 0.789 0.696d Dipole momente/10 30 C m 14.9 10.1 15.0 8.9 ma 5.4 10.3 5.7 11.4 mb f 0.8 0.0 1.0 0.0f mc mtot 15.8 14.4 16.1 14.5 Energy differenceg/kJ mol 1 DE 0.0h 6.2 0.0i 4.5 Basis set: 6-311++G(3df,3pd). b This work. c A-reduction.16 Fixed at this value in the least-squares fit. e 1 debye = 3.33564 10 30 C m. f For symmetry reasons. g Corrected for zero-point vibrational energy. h Electronic energy: 6857 160.41 kJ mol 1. i Electronic energy: 6851 642.59 kJ mol 1. a d to those discussed above for H2CQCQCHSeCN. The dihedral angles are also similar in the two calculations with the exception of the dihedral angles related to the selenocyanate group, whose orientation relative to the ring, as expressed by the H4–C1–Se9–C10 dihedral angle, differs by 5.61 in the two methods of calculation. ap has a symmetry plane (Cs symmetry) with the selenocyanate group lying in the symmetry plane. Interestingly, the C–C bonds of the ring adjacent to this group (the C1–C2 and C1–C3 bonds) are shorter by about 2 pm (Table 3) than the bond length opposite to the selenocyanate group (the C2–C3 bond) in both methods of calculation. This difference in bond lengths is typical for substituted cyclopropanes with Cs symmetry.20 It is noted that the C–C bond lengths of sc, which has no symmetry, do not show (Table 3) this kind of variation. fundamentals. The quantum chemical calculations above indicate that there is one fundamental below 50 cm 1 (the C–S torsion) and six additional between 100 and 500 cm 1 (not given in Table 1 or 2) both for ac and for sp. Another factor that contributes negatively to the intensity is the fact that selenium has six naturally occurring isotopes, of which five are relatively abundant (76Se (9.0%), 77Se (7.6%), 78 Se (23.5%), 80Se (49.8%), and 82Se (9.2%)), which means that the intensity is reduced accordingly. The presence of relatively large concentrations of more than one rotameric form would have a similar effect on the intensity. Finally, the 14 N nucleus (99.6% abundance) has a spin = 1, and the nuclear quadrupole coupling effects will therefore split each transition into several components, which were not resolved in our experiment, but will broaden the lines and reduce peak intensities. The theoretical predictions shown in Table 2 indicate that the parent 80Se isotopologue of the ac rotamer is a prolate asymmetrical top with Ray’s asymmetry parameter21 k E 0.91, with ma as its major dipole moment component (Table 2). Accumulations of aR-branch transitions separated by roughly B + C E 2.4 GHz were therefore expected in the 48–80 GHz spectral region. The high-K 1 members of these series would be modulated at comparatively low Stark fields, which would facilitate their assignments. Series of accumulations were readily seen to protrude from the background of weaker transitions, when a relatively low Stark field was applied. A typical example is the accumulation associated with the ground vibrational state of the J = 28 ’ 27 a-type transitions of the 80Se species, which is shown in Fig. 5. These accumulations were the key to the assignment of the MW spectrum of ac. It was found that pairs of aR-lines with Microwave spectrum and assignment of the spectrum of the ac sof H2CQCQCH80SeCN Survey spectra taken at about 0 1C revealed a comparatively weak and dense spectrum, with relatively broad absorption lines occurring every few MHz throughout the 48–80 GHz spectral interval. Several factors contribute to the intensity of a MW spectrum. The intensity is proportional to the square of the principal-axis dipole moment components and inversely proportional to the partition function.17 sp has a comparatively large mb, and ac has a large ma (Table 2). The fact that a relatively weak spectrum was observed must therefore indicate that the partition function is relatively large at 0 1C, which causes a low population in each quantum state, and consequently a weak spectrum. The large value of the partition function is primarily caused by relatively small rotational constants (Table 2) and several low-frequency vibrational 4142 | Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 Fig. 5 This portion of the MW spectrum shows the a-type J = 28 ’ 27 of the ground vibrational state of the H2CQCQCH80SeCN isotopologue of the ac conformer. The values of the coalescing K 1—lines are shown above each assigned line. The K 1 = 12 and 14, and the K 1 = 11 and 15 overlap. The spectrum was recorded applying a Stark-modulation field strength of approximately 120 V cm 1. This journal is c the Owner Societies 2008 Table 5 Spectroscopic constantsa,b of ac of H2CQCQCHSeCN Species: H2CQCQCH80SeCN H2CQCQCH78SeCN Vibrational state: Ground 1st ex. torsion 1st bending Ground A/MHz B/MHz C/kHz DJ/kHz DJK/kHz DK/kHz dJ/kHz dK/kHz FdJK/Hz Rmse No. transitionsf 5487.4(56) 1325.453(81) 1129.332(89) 0.6970(41) 16.141(21) 190c 0.299(17) 0.993c 0.234(13) 1.6350 156 5614.39(81) 1334.609(86) 1130.704(87) 0.8551(43) 17.819(20) 190c 0.160(18) 0.993c 0.148(14) 1.1929 173 5638(14) 1316.25(16) 1125.08(17) 0.6549(56) 15.648(31) 190c 0.134(22) 0.993c 0.133(21) 1.6092 139 5536.2c 1326.358(38) 1132.194(44) 0.7200(40) 16.679(54) 190c 0.229c 0.993c 0.272(37) 1.2503 62 a A-Reduction, Ir-representation.16 Spectra are found in the ESI, Tables 1S–4S. b Uncertainties represent one standard deviation. c Fixed; see text. d Further sextic constants preset at zero. e Root-mean-squares deviation. f Number of transitions used in the weighted least-squares fit. identical K 1 Z 7 coalesce in this spectral region, because k E 0.91, and that transitions with different values of K 1 frequently overlap. A total of 156 aR-lines with K 1 Z 5 were ultimately assigned. Definite assignments of transitions with K 1o5 could not be made unambiguously, primarily because these transitions have slow Stark effects and are therefore difficult to modulate, and they are often overlapped. Searches for b-type lines were also made because a mb component of about 6 10 30 C m is predicted for ac (Table 2), but no such transitions could be assigned. This is not surprising because the b-type transitions should be considerably weaker than the already weak aR-lines because ma is calculated to be more that twice as large as mb (Table 2). The aR-spectrum of ac was fitted to Watson’s A-reduction Hamiltonian using the Ir-representation16 employing Sørensen’s program Rotfit.22 The inverse squares of the estimated uncertainties of the transitions were used as weights in the least-squares fitting procedure. The spectrum is shown in Table 1S in the ESIw and the spectroscopic constants of the 80 Se isotopologue are listed in Table 5, and repeated in Table 2 for convenient comparison with the theoretical predictions. The assigned aR-lines provide insufficient information for an accurate determination of the DK and dK quartic centrifugal distortion constants for this near-prolate rotor. These two centrifugal distortion constants were held fixed at the B3LYP values (Table 2) in the weighted least-squares fit. One of the sextic centrifugal distortion constants (FJK) could also be determined, as shown in Table 5. The assignment of the ground-state spectrum of the 78Se isotopologue was straightforward. The spectrum consisting of 62 transitions is listed in Table 3S in the ESIw and the spectroscopic constants are given in Table 5. The A rotational constant was fixed at 5536.2 MHz in this case. This value was estimated from the B3LYP structure. Attempts to assign the spectra of the 76Se (9.0%) and 82Se (9.2%) species were made, but these spectra were found to be so weak that a detailed assignment could not be achieved. Excited states of the 80 Se isotopologue of ac The MW spectra of the first excited state of the torsion about the C2–Se7 bond and the first excited state of the lowest This journal is c the Owner Societies 2008 bending vibration were assigned for the 80Se species. The spectra are listed in Tables 2S and 3S in the ESI,w respectively, and the spectroscopic constants are displayed in Table 5. It is possible to calculate the vibration–rotation interaction constants (the a’s)17 defined by aX = X0 X1, where X0 are the ground-state rotational constants and X1 are the rotational constants of a particular excited vibrational state. The entries in Table 5 yield aA = 127(6), aB = 9.16(12), and aC = 1.37(12) MHz for the torsional state, and aA = 151(14), aB = 9.20(17), and aC = 4.25(19) MHz for the bending vibration. The corresponding B3LYP values are –40.84, –3.93, and –1.72 MHz, respectively, for the torsion, and –159.14, 8.23, and 4.16 MHz, respectively, for the bending vibration. The agreement is far from perfect, but the a’s are derived by taking the third derivatives of the potential energy at the minimum, which is not expected to be very accurate in the present case. Only rough relative intensity measurements of the two lowest vibrational fundamentals could be performed in this case owing to the density of the spectrum. These measurements yielded 62(30) and 87(40) cm 1 for the torsion and bending vibrations, respectively. The B3LYP values corrected for anharmonicity are 29 and 107 cm 1 for these vibrations. Structure of ac It is of interest to compare the experimental rotational constants (Tables 2 and 5) of the 80Se isotopologue with the theoretical counterparts (Table 2). The B3LYP rotational constants A, B, and C differ from the experimental rotational constants of the ground vibrational state by 2.5, 1.9, and 1.3%, respectively, whereas the MP2 rotational constants differ by 5.0, 1.6, and 0.2%, respectively. The theoretical rotational constants are approximations of the equilibrium rotational constants, whereas the experimental rotational constants are effective constants. This makes a comparison somewhat difficult. However, an agreement to within 2%, or better, is expected. The largest differences, namely 2.5% (B3LYP) and 5.0% (MP2), are seen for the A rotational constant. This constant depends much on the value of the C1–C2–Se7–C8 dihedral angle. The B3LYP value of 130.91 for this dihedral angle (Table 1) seems to be too large because the rotational constants obtained from this structure indicate that Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 | 4143 the B3LYP structure is more prolate than the experimental rotational constants imply. Similarly, the MP2 structure with a dihedral angle of 126.01 seems too oblate. Our best estimate is 129(5)1 for this dihedral angle. This value is very different from the corresponding value of the C–C–Se–C dihedral angle in the anticlinal conformer of H2CQCHSeCN, which is 166(3)1.4 Interestingly, the anticlinal conformer of the sulfur analogue, H2CQCQCHSCN, has a C–C–S–C dihedral angle of 1341,23 almost the same as that found in ac (129(5)1). Searches for sp This rotamer is predicted to be favoured in the MP2 calculations, whereas the opposite was found using the B3LYP procedure (Table 2). The statistical weight of sp is 1 compared to 2 for ac. The b-type spectrum of sp was expected to be much stronger than the a-type spectrum, because mb is the predominating dipole moment component (Table 2). The b-type spectra are generally more difficult to assign than the a-type spectra. Extensive searches were made for both the a- and the b-type MW spectra of this form, but they were not found. It is likely that this rotamer is a high-energy form of the molecule. Interestingly, a similar conclusion was made in the case of the sulfur counterpart, H2CQCQCHSCN, where the anticlinal form is at least 2 kJ mol 1 more stable than the synperiplanar rotamer.23 The reduced stability of the synperiplanar form of H2CQCQCHSCN relative to the anticlinal conformer was explained as largely caused by non-bonded repulsion between the p electrons of the H2CQC double bond and the p electrons of the triple CRN bond.23 It is possible that a similar kind of interaction dictates a lower stability of sp compared to ac in the present case of H2CQCQCHSeCN. dominated by transitions originating from sc. This prolate rotamer (k E 0.66) is predicted (Table 4) to have its major dipole-moment component along the a-axis, and accumulations of aR-lines, similar to those seen for H2CQCQCHSeCN and separated by roughly B + C E 3.2 GHz, were therefore expected. The observed spectrum was indeed comparatively weak and consisted of accumulations of lines, as predicted. Only very weak lines were seen between the accumulation regions, which have a complicated fine structure extending over several hundred MHz because the ground-vibrational and excitedstate spectra contribute together with the spectra of the isotopologues. The ground-state aR-spectrum was readily assigned. The btype lines are predicted to be much weaker because mb E 1/3 ma (Table 4), and none of them were identified unambiguoulsy. A total of 161 transitions were assigned for the ground state, whose spectrum is given in Table 5S in the ESI,w while the spectroscopic constants are listed in Table 6 and repeated in Table 4 for convenient comparison with the theoretical counterparts. Only two of the quartic centrifugal distortion constants (DJ and DJK) were determined, whereas the remaining quartic constants were kept fixed at the B3LYP values. No sextic constants were fitted in this case. The assignment of the ground-state spectrum of the 78Se isotopologue was straightforward. The spectrum consisting of 99 transitions is listed in Table 9S in the ESIw and the spectroscopic constants are given in Table 6. Attempts to assign the spectra of the 76Se (9.0%) and 82Se (9.2%) species were made, but these spectra were found to be so weak that a detailed assignment could not be achieved. Excited states of the Microwave spectrum and assignment of the spectrum of sc of C3H5SeCN The MW spectrum of this compound was expected to be relatively weak and to consist of broad spectral lines for reasons similar to those discussed above for H2CQCQCHSeCN. The B3LYP and MP2 calculations predict (Table 4) that sc is more stable than ap by 6.2 and 4.5 kJ mol 1, respectively. The statistical weight of sc is twice that of ap. It was therefore expected that the MW spectrum would be Table 6 80 Se isotopologue of sc The MW spectra of the first excited state of the torsion about the C2–Se9 bond and the first excited state of the lowest bending vibrations were assigned for the 80Se species. The spectrum of an additional excited state, which may be the second lowest bending vibration, was also assigned. The spectra are listed in Tables 6S–9S in the ESIw and the spectroscopic constants are displayed in Table 6. It was not possible to determine experimentally the A rotational constants of the vibrationally excited states. These Spectroscopic constantsa,b of sc of C3H5SeCN Species: C3H580SeCN C3H578SeCN Vibrational state: Ground 1st ex. torsion Lowest bending Low bendingc Ground A/MHz B/MHz C/kHz DJ/kHz DJK/kHz DK/kHz dJ/kHz dK/kHz Rmse No. transitionsf 4176.4(19) 1812.965(96) 1338.271(99) 1.2605(29) 9.5769(87) 30.7d 0.407d 0.696d 1.6787 161 4171.62d 1810.869(31) 1340.066(42) 1.1490(60) 8.983(20) 30.7d 0.407d 0.696d 2.2360 85 4160.7d 1810.556(38) 1339.289(51) 1.2890(73) 9.664(28) 30.7d 0.407d 0.696d 2.1992 89 4176.4d 1820.090(84) 1335.63(10) 1.106(23) 9.842(54) 30.7d 0.407d 0.696d 3.2402 32 4219.7(69) 1814.67(33) 1341.93(34) 1.2662(63) 9.723(21) 30.7d 0.407d 0.696d 2.2214 99 A-Reduction, Ir-representation.16 Spectra are found in the ESI, Tables 4S–9S. b Uncertainties represent one standard deviation. c Tentative assignment; see text. d Fixed; see text. e Root-mean-squares deviation. f Number of transitions used in the weighted least-squares fit. a 4144 | Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 This journal is c the Owner Societies 2008 constants were therefore kept fixed in the least-squares fit. An estimate of the A rotational constants of the first excited state of the torsion and of the lowest bending vibration were made by adding the aA’s obtained in the B3LYP calculations to the A rotational constant of the ground vibrational state. The B3LYP values were aA = –4.75 MHz for the torsion and 15.73 MHz for the lowest bending vibration. The A rotational constant of the ground state was employed for the last vibration, because we are not sure what excited state this is. Rough relative intensity measurements yielded 65(30) and 92(40) cm 1 for the torsion and bending vibrations, respectively. The B3LYP values corrected for anharmonicity are 53 and 109 cm 1, respectively, for these two vibrations. Structure of sc The B3LYP rotational constants A, B, and C differ from the experimental rotational constants of the ground vibrational state by 3.9, 4.6, and 2.4%, respectively, whereas the MP2 rotational constants differ by 1.9, 1.8, and 0.6%, respectively. Presumably, this reflects the fact that the MP2 structure is somewhat more accurate than the B3LYP structure. The rotational constants depend much on the orientation of the selenocyanate group. The H4–C1–Se9–C10 dihedral angle is assumed to be closer to the MP2 value (81.61; Table 3) than to the 76.01 obtained in the B3LYP calculations. Our best estimate of this angle is 80(4)1. Searches for the spectrum of ap This very asymmetric (k E 0.1) conformer is predicted to have a relatively large ma and searches were consequently made for its aR-type lines, which would be stronger than any other transitions. There are practically no strong lines between the accumulation regions, where many of the lines belonging to the hypothetical ap should occur. It is therefore concluded that this rotamer must be a high-energy form of the molecule, which must be at least 3 kJ mol 1 less stable than sc. This is in agreement with the theoretical predictions (Table 4). The preferred conformer of C3H5SeCN has a synclinal orientation of the H–C–Se–C chain of atoms. There are few examples that can be used for comparison with the present conformational results for C3H5SeCN, but it is noted that the preferred form of cyclopropaneselenol, C3H5SeH, has a synclinal conformation for the H–C–Se–H link of atoms, according to our MW study.24 methods agree that one of the conformers has an exact synperiplanar conformation (the C–C–Se–C dihedral angle = 01). The other anticlinal form is predicted to have a C–C–Se–C dihedral angle of 126.01 (MP2), or 130.91 (B3LYP). The synperiplanar conformer is slightly less stable by 1.3 kJ mol 1, according to the MP2 calculations. The B3LYP method finds the opposite, namely that the synperiplanar form is less stable than the anticlinal rotamer by 3.1 kJ mol 1. The anticlinal form was found in the MW spectrum and is shown to be the preferred form of the molecule, in agreement with the B3LYP predictions. The C–C–Se–C dihedral angle was determined to be 129(5)1 by comparison of the theoretical and experimental rotational constants. The conformational behaviour of H2CQCQCHSeCN is strikingly different from its congener H2CQCHSeCN, which prefers a synperiplanar form by 4.5(4) kJ mol 14 over an anticlinal form and whose C–C–Se–C dihedral angle is more than 301 larger than in H2CQCQCHSeCN. For C3H5SeCN, the two theoretical methods predict that the conformer having a synclinal (E 801) orientation of the H–C–Se–C chain of atoms is preferred by several kJ mol 1 over an antiperiplanar form. The MW spectrum of the synclinal conformer has been assigned. There is no indication in the spectrum for substantial amounts of a second antiperiplanar form, which must be less stable than the synclinal rotamer by at least 3 kJ mol 1. The H–C–Se–C dihedral angle was determined to be 80(4)1 in the identified synclinal conformer. A comparison with the oxygen (R-OCN) and sulfur (R-SCN) analogues of the two title compounds would have been of interest. However, only one such sulfur analogue, H2CQCQCHSCN, is known to date, and this compound has conformational properties very similar to those of H2CQCQCHSeCN, as pointed out above. Acknowledgements We thank Anne Horn for her skilful assistance. The Research Council of Norway (Program for Supercomputing) is thanked for a grant of computer time. R.M. thanks the Research Council of Norway for financial assistance through Contract 177540/V30. J.-C.G. thanks the University of Oslo for a travel grant and the ‘‘PID-OPV’’ (CNRS) for financial support. Notes and references 4. Conclusions The microwave spectra of H2CQCQCHSeCN and C3H5SeCN have been investigated for the first time. The ground vibrational state spectrum of the 80Se and 78Se isotopologues of one conformer of each of these two compounds has been assigned together with several vibrationally excitedstate spectra of their 80Se isotopologues. The spectroscopic work has been augmented by B3LYP and MP2 quantum chemical calculations employing the 6-311++G(3df,3pd) basis set. The B3LYP and MP2 methods both predict the existence of two conformers for H2CQCQCHSeCN, which can be characterized by the value of the C–C–Se–C dihedral angle. Both This journal is c the Owner Societies 2008 1. T. Sakaizumi, Y. Kohri, O. Ohashi and I. Yamaguchi, Bull. Chem. Soc. Jpn., 1978, 51, 3411. 2. T. Sakaizumi, M. Obata, K. Takahashi, E. Sakaki, Y. Takeuchi, O. Ohashi and I. Yamaguchi, Bull. Chem. Soc. Jpn., 1986, 59, 3791. 3. T. Sakaizumi and T. Itakura, J. Mol. Spectrosc., 1994, 163, 1. 4. H. Møllendal and J.-C. Guillemin, J. Phys. Chem. A, 2007, 111, 7073. 5. E. H. Riague and J.-C. Guillemin, Organometallics, 2002, 21, 68. 6. G. Bajor, T. Veszprémi, E. H. Riague and J.-C. Guillemin, Chem.–Eur. J., 2004, 10, 3649. 7. J.-C. Guillemin, G. Bajor, E. H. Riague, B. Khater and T. Veszprémi, Organometallics, 2007, 26, 2507. 8. B. Khater, J.-C. Guillemin, G. Bajor and T. Veszprémi, Inorg. Chem., 2008, 47, 1502. 9. H. Møllendal, A. Leonov and A. de Meijere, J. Phys. Chem. A, 2005, 109, 6344. Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 | 4145 10. H. Møllendal, G. C. Cole and J.-C. Guillemin, J. Phys. Chem. A, 2006, 110, 921. 11. F. J. Wodarczyk and E. B. Wilson Jr, J. Mol. Spectrosc., 1971, 37, 445. 12. M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, 4146 | Phys. Chem. Chem. Phys., 2008, 10, 4138–4146 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, in GAUSSIAN 03, revision B.03, Pittsburgh, PA, 2003. C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618. A. D. Becke, Phys. Rev. A, 1988, 38, 3098. C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785. J. K. G. Watson, in Vibrational Spectra and Structure, ed. J. R. Durig, Elsevier, 1977. W. Gordy and R. L. Cook, in Techniques of Chemistry, Vol XVII: Microwave Molecular Spectra, ed. A Weissberger, John Wiley & Sons, 1984. T. H. Dunning Jr, J. Chem. Phys., 1989, 90, 1007. Y. Endo, M. C. Chang and E. Hirota, J. Mol. Spectrosc., 1987, 126, 63. R. E. Penn and J. E. Boggs, J. Chem. Soc., Chem. Commun., 1972, 666. B. S. Ray, Z. Physik, 1932, 78, 74. G. O. Sørensen, in ROTFIT. Personal communication, 1972. H. Møllendal, G. C. Cole and J.-C. Guillemin, J. Phys. Chem. A, 2007, 111, 2542. R. Mokso, H. Møllendal and J. C. Guillemin, J. Phys. Chem. A, 2008, submitted. This journal is c the Owner Societies 2008