1.5 Fundamental physical constants The following values were
advertisement

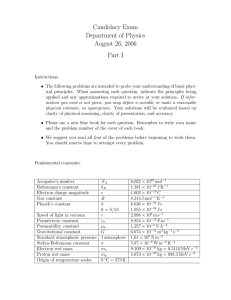
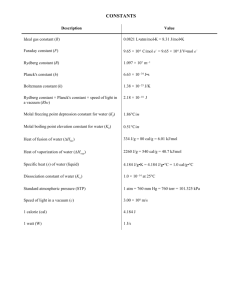
1.5 Fundamental physical constants The following values were recommended by the CODATA Task Group on Fundamental Constants in 1986. For each constant the standard deviation uncertainty in the least significant digits is given in parentheses. Quantity Symbol Value permeability of vacuum speed of light in vacuum 1 permittivity of vacuum Planck constant µ0 c0 2 ε0 = 1/µ0c0 h h = h / 2π elementary charge electron rest mass proton rest mass neutron rest mass atomic mass constant, (unified atomic mass unit) Avogadro constant Boltzmann constant Faraday constant gas constant zero of the Celsius scale molar volume, ideal gas, p = 1 bar, θ = 0 °C standard atmosphere Bohr radius Rydberg constant Bohr magneton electron magnetic moment e me mp mn mu = 1 u 4π×10 H m (defined) -1 299 792 458 m s (defined) -12 -1 8.854 187 816 ...×10 F m -34 6.626 075 5(40) ×10 J s -34 1.054 572 66(63)×10 J s -19 1.602 177 33(49)×10 C -31 9.109 389 7(54)×10 kg -27 1.672 623 1(10)×10 kg -27 1.674 928 6(10)×10 kg -27 1.660 540 2(10)×10 kg 1 (1) -1 -2 2 -2 -7 -1 23 -1 L, NA k F R 6.022 136 7(36)×10 mol -23 -1 1.380 658 (12)×10 J K 4 -1 9.648 530 9(29)×10 C mol -1 -1 8.314 510 (70) J K mol 273.15 K (defined) -1 22.711 08(19) L mol atm 2 2 a0 = 4πε0h /mee R∞ = Eh/2hc0 µB = eh/2me µe 101 325 Pa (defined) -11 5.291 772 49(24)×10 m 7 -1 1.097 373 153 4(13)×10 m -24 -1 9.274 015 4(31)×10 J T -24 -1 9.284 770 1(31)×10 J T -1 2 -1 -1 H m = N A = N s C ; F m = C J m ; ε0 may be calculated exactly from the defined values of µ0 and c0. Chapter 1 - 1 Quantity Symbol Value Landé g-factor for free electron nuclear magneton proton magnetic moment proton magnetogyric ratio magnetic moment of ge = 2µe/µB µN = (me/mp)µB µp γp µ p′ /µB 2.002 319 304 386 (20) -27 -1 5.050 786 6(17)×10 J T -26 -1 1.410 607 61(47)×10 J T 8 -1 -1 2.675 221 28(81)×10 s T -3 1.520 993 129(17)×10 proton resonance γ p′ 42.576 375(13) MHz T frequency per field in H2O Stefan-Boltzmann constant first radiation constant second radiation constant gravitational constant standard acceleration of free fall σ / 2π k /15h c0 2 c1 = 2πhc0 c2 = hc0/k G gn protons in H2O, µ p′ -1 5 4 3 2 -8 -2 -4 5.670 51(19)×10 W m K -16 2 3.741 774 9(22)×10 W m -2 1.438 769(12)×10 m K -11 3 -1 -2 6.672 59(85)×10 m kg s -2 9.806 65 m s (defined) Values of common mathematical constants Mathematical constant Symbol Value ratio of circumference to diameter of a circle base of natural logarithms natural logarithm of 10 π e ln 10 3.141 592 653 59 2.718 281 828 46 2.302 585 092 99 Reference: Cohen, E.R. and Taylor, B.N., The 1986 Adjustment of the Fundamental Physical Constants, CODATA Bull. 63 (1986) 1-49. Chapter 1 - 2