Microwave and Quantum Chemical Study of Allyldifluorosilane (H C SiF H)
advertisement

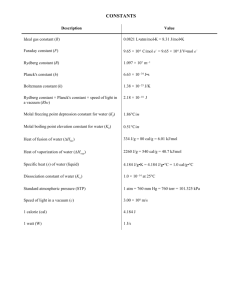
6608 J. Phys. Chem. A 2010, 114, 6608–6612 Microwave and Quantum Chemical Study of Allyldifluorosilane (H2CdCHCH2SiF2H) Harald Møllendal,*,† Svein Samdal,† Gamil A. Guirgis,‡ and Charles J. Wurrey§ Centre for Theoretical and Computational Chemistry (CTCC), Department of Chemistry, UniVersity of Oslo, P.O. Box 1033 Blindern, NO-0315 Oslo, Norway, Department of Chemistry and Biochemistry, College of Charleston, Charleston, South Carolina 29424, Department of Chemistry, UniVersity of MissourisKansas City, Kansas City, Missouri 64110 ReceiVed: March 4, 2010; ReVised Manuscript ReceiVed: April 14, 2010 The microwave spectrum of allyldifluorosilane (H2CdCHCH2SiF2H) has been investigated for the first time in the 28-80 GHz spectral interval at a temperature of -30 °C. The spectrum of the ground vibrational state of one conformer characterized by an anticlinal orientation for the CdC-C-Si chain of atoms and a synclinal conformation for the CsCsSisH link has been assigned. This rotamer was found to be at least 2 kJ/mol more stable than further rotameric forms. The spectroscopic investigation has been augmented with quantum chemical calculations employing the MP2 and B3LYP methods using the 6-311++G(3df,3pd) basis set. The theoretical predictions are generally in good agreement with the experimental results. Introduction Recently, there has been great interest in the syntheses and investigation of the spectroscopic, conformational, and structural properties of silicon-containing compounds. Recent examples include F3SiCH2CH2SiF3,1 cyclopropylmethylsilane,2,3 ClCH2SiH3,4 Cl2CHSiH3,4 ClCH2SiF3,4 Cl2CHSiF3,4 diethylsilane,5 diethyldifluorosilane,5 vinyl silyl fluoride,6 H3SiCH2SiH2CH3,7 disilabutane,8 silacyclopentane,9 and ethylmethylfluorosilane.10 In this work, we focus on the little-investigated conformational and structural properties of compounds possessing the difluorosilane (-SiF2H) group, with allyldifluorosilane (H2CdCHCH2SiF2H) as an example. Previous work on molecules with the difluorosilane group includes spectroscopic investigations of difluorosilane (SiH2F2),11-13 methyl difluorosilane (H3C-SiF2H),14,15 and vinyldifluorosilane (H2CdCHSiF2H).16 Only the last-mentioned of these compounds is capable of displaying rotational isomerism. This property can perhaps be best envisaged by reference to the hydrogen atom of the difluorosilane group. The SisH bond is synperiplanar (sp; CdCsSisH dihedral angle ) 0°) with the vinyl group in one form, and anticlinal (ac; approximately 120° from synperiplanar) in the second rotamer. An infrared study of this compound dissolved in krypton yielded an energy difference of 1.42(14) kJ/mol, with the sp form as the more stable.17 Only the sp rotamer was found in a microwave (MW) investigation of the gaseous species, and this form was shown to be at least 3 kJ/mol more stable than the ac conformer.16 In the present work, these studies are extended to include the synthesis and MW study augmented with quantum chemical calculations of a novel member of this class of compounds, namely, allyldifluorosilane (H2CdCHCH2SiF2H). Studies of other allylsilane compounds such as the parent allylsilane (H2CdCHCH2SiH3)18-20 and allyltrifluorosilane (H2CdCHCH2SiF3)21 have been reported. It was concluded in * To whom correspondence should be addressed. Tel: +47 2285 5674. Fax: +47 2285 5441. E-mail: harald.mollendal@kjemi.uio.no. † University of Oslo. ‡ College of Charleston. § University of MissourisKansas City. these studies that there are two rotameric forms of these compounds, which can be characterized by the CdCsCsSi dihedral angle. The preferred form of these two compounds has an ac orientation of this angle, which has been determined to be 106.8(11)° in allylsilane.19 There is also considerable evidence that a high-energy sp conformer coexists with the ac rotamer in these two compounds.18-20 The conformational problem posed by the title compound is more complicated than for H2CdCHCH2SiH3 and H2CdCHCH2SiF3 because of the asymmetry of the SiF2H group. Two dihedral angles, namely, the CdCsCsSi and CsCsSisH dihedral angles, can now be conveniently used to describe the conformational properties of H2CdCHCH2SiF2H. Five rotamers may exist as minima on the conformational energy hypersurface. These forms are sketched in Figure 1 and atom numbering is given on the conformer denoted I. The CdCsCsSi chain of atoms has an +ac conformation (roughly +120°) in I, II, and III depicted in Figure 1, and a sp conformation in IV and V. The CsCsSisH link is +synclinal (+sc) (about +60°) in I and V, antiperiplanar (ap) (roughly 180°) in II and IV, and -ac in III. A mirror image conformation exists for each of I, II, III, and V. A successful investigation of a delicate conformational equilibrium such as the one presented by H2CdCHCH2SiF2H requires experimental methods possessing high resolution. MW spectroscopy meets this requirement because of its superior accuracy and resolution, making this method especially well suited for conformational studies of gaseous species. The spectroscopic work has been augmented by high-level quantum chemical calculations, which were conducted with the purpose of obtaining information for use in assigning the MW spectrum and investigating properties of the potential-energy hypersurface. Experimental Section Synthesis. The allyldifluorosilane sample was prepared by fluorination of the corresponding dichlorosilane using a freshly sublimed sample of antimony trifluoride without the use of a solvent. Allyldichlorosilane was obtained as a byproduct from the chlorination of allylsilane by tin tetrachloride. A manuscript describing this reaction is in preparation. The progress of the 10.1021/jp101950z 2010 American Chemical Society Published on Web 05/26/2010 Allyldifluorosilane J. Phys. Chem. A, Vol. 114, No. 24, 2010 6609 Figure 1. Models of H2CdCHCH2SiF2H with atom numbering. The MW spectrum of conformer I was assigned. I was found to be at least 2 kJ/mol more stable than the other rotameric forms. MP2 and B3LYP calculations predict I to be favored by ∼2-10 kJ/mol relative to II, III, and IV. The theoretical calculations predict that V is a transition state. fluorination reaction was monitored by taking samples every 5 min for infrared spectroscopy to observe the disappearance of the dichloro compound. The final sample was purified by trapto-trap distillation three times. The identity of the compound was checked by NMR and infrared spectroscopy. Microwave Experiment. The spectrum of H2CdCHCH2SiF2H was studied in the 28-80 GHz frequency interval by Stark-modulation spectroscopy using the microwave spectrometer of the University of Oslo. Details of the construction and operation of this device have been given elsewhere.22,23 This spectrometer has a resolution of about 0.5 MHz and measures the frequency of isolated transitions with an estimated accuracy of ≈0.10 MHz. The experiments were performed at about -30 °C by cooling the MW cell with dry ice in an attempt to increase the intensity of the spectrum, which was recorded at a pressure of roughly 10 Pa. Quantum Chemical Methods. The present ab initio and density functional theory (DFT) calculations were performed employing the Gaussian 03 suite of programs,24 running on the Titan cluster in Oslo. Electron correlation was taken into consideration in the ab initio calculations using Møller-Plesset second-order perturbation calculations (MP2).25 Becke’s threeparameter hybrid functional26 employing the Lee, Yang, and Parr correlation functional (B3LYP)27 was employed in the density functional theory (DFT) calculations. The 6-311++G(3df,3pd) wave function, which is of triple-ζ quality and augmented with diffuse functions, was used in both MP2 and B3LYP calculations. Geometry optimizations with no symmetry restraints were performed on the stationary points found for H2CdCHCH2SiF2H employing the default convergence criteria of Gaussian 03. Results and Discussion Quantum-Chemical Calculations. The energies, structures, rotational and centrifugal distortion constants, harmonic vibrational frequencies, and dipole moments were calculated for conformers I-IV by employing both the MP2 and B3LYP procedures. Only positive vibrational frequencies were calcu- lated for each of these conformers, which are therefore minima on the potential energy hypersurface. Interestingly, one negative vibrational frequency associated with the torsion about C4-C6 bond was calculated for V in both the MP2 and B3LYP calculations, which therefore implies a first-order transition state. Calculations of the anharmonic frequencies are rather costly, and these constants were calculated only for I (whose MW spectrum was assigned) using the B3LYP procedure. The MP2 structures of I-IV are shown in Table 1, while the corresponding B3LYP structures are collected in Table 1S in the Supporting Information. The rotational constants calculated from the MP2 structures are shown in Table 2, together with Watson’s A-reduction quartic centrifugal distortion constants,28 the components of the dipole moment along the principal inertial axes, and the energy differences relative to the energy of the global minimum conformer, which turned out to be I. The energy differences have been corrected for zeropoint vibrational energies. Corresponding B3LYP parameters are listed in the Supporting Information, Table 2S. The MP2 and B3LYP structures of the transition state V are displayed in Table 3S. This rotameric form has an electronic energy that is 13.0 kJ/mol above the energy of I in the MP2 calculations, and 11.8 kJ/mol above I in the B3LYP calculations. The harmonic and anharmonic vibrational frequencies of this conformer are listed in Table 4S. Both the MP2 (Table 2) and B3LYP (Table 2S) calculations predict that I is the preferred form of the molecule by ∼2-3 kJ/mol relative to the two other CdCsCsSi ac conformers (II and III), and about 10 kJ/mol more stable than the CdC-C-Si sp rotamer (IV). The high energy of the CdCsCsSi sp arrangement relative to the ac conformation is reminiscent of the findings made for H2CdCHCH2SiH318-20 and H2CdCHCH2SiF3.21 The structures in Tables 1 and 1S reveal that the bond lengths and bond angles are rather similar. A notable difference is seen for the C1dC4sC6sSi9 dihedral angles, which are less by ∼5° 6610 J. Phys. Chem. A, Vol. 114, No. 24, 2010 Møllendal et al. TABLE 1: MP2/6-311++G(3df,3pd) Structures of Four Conformers of H2dCHCH2SiF2H Ia II C1-H2 C1–H3 C1–C4 C4–H5 C4–C6 C6–H7 C6–H8 C6–Si9 Si9–H10 Si9–F11 Si9–F12 Bond Length (pm) 108.2 108.2 108.0 108.0 133.6 133.5 108.5 108.4 149.8 149.9 109.1 109.3 109.2 109.2 185.1 185.1 146.3 146.4 159.9 159.9 159.9 159.6 H2–C1–H3 H2–C1–C4 H3–C1–C4 C1–C4–H5 C1–C4–C6 H5–C4–C6 C4–C6–H7 C4–C6–H8 C4–C6–Si9 H7–C6–H8 H7–C6–Si9 H8–C6–Si9 C6–Si9–H10 C6–Si9–F11 C6–Si9–F12 H10–Si9–F11 H10–Si9–F12 F11–Si9–F12 Angles 117.7 121.3 121.1 118.9 124.5 116.5 111.5 110.8 109.3 107.9 109.1 108.2 113.4 110.6 109.1 108.2 108.2 107.2 H2–C1–C4–H5 H2–C1–C4–C6 H3–C1–C4–H5 H3–C1–C4–C6 C1–C4–C6–H7 C1–C4–C6–H8 C1–C4–C6–Si9 H5–C4–C6–H7 H5–C4–C6–H8 H5–C4–C6–Si9 C4–C6–Si9–H10 C4–C6–Si9–F11 C4–C6–Si9–F12 H7–C6–Si9–H10 H7–C6–Si9–F11 H7–C6–Si9–F12 H8–C6–Si9–H10 H8–C6–Si9–F11 H8–C6–Si9–F12 a (deg) 117.7 121.3 121.0 119.0 124.4 116.5 111.0 110.8 111.8 107.4 107.8 108.0 114.5 109.2 109.7 107.9 107.8 107.5 Dihedral Angle (deg) 179.8 179.6 2.8 1.8 –0.7 –0.4 –177.7 –178.2 –138.6 –134.1 –18.4 –15.0 100.7 105.6 44.4 48.1 164.5 167.2 –76.3 –72.3 –61.5 174.9 176.8 53.8 59.1 –63.8 176.4 52.8 54.6 –68.4 –63.0 174.0 59.3 –62.9 –62.5 175.9 179.9 58.3 III IV TABLE 2: MP2/6-311++G(3df,3pd) Parameters of Spectroscopic Interest of Four Conformersa of H2CdCHCH2SiF2H Ib 108.2 108.0 133.5 108.5 149.9 109.2 109.2 185.1 146.4 159.7 159.9 108.2 108.0 133.5 108.5 150.3 109.6 109.6 184.8 146.4 159.8 159.8 117.7 121.2 121.0 118.7 124.6 116.7 111.1 110.9 110.1 107.7 108.8 108.1 114.0 109.1 110.2 108.0 108.1 107.1 117.0 122.5 120.5 118.2 126.1 115.7 109.7 109.7 118.5 104.7 106.7 106.7 112.3 110.9 110.9 107.7 107.7 106.9 179.7 2.1 –0.5 –178.1 –139.4 –19.7 100.0 42.9 162.7 –77.7 50.0 –70.9 171.7 –72.0 167.1 49.7 171.3 50.4 –67.0 180.0 0.0 0.0d 180.0 122.8 –122.8 0.0 –57.2 57.2 180.0 180.0 59.4 –59.4 55.7 –64.9 176.0 –55.7 –176.4 65.9 The MW spectrum of this conformer was assigned. in the MP2 structures of IsIII compared to the corresponding B3LYP structures. Comparison with experimental findings for similar molecules is warranted. There is an accurate estimate of the equilibrium structure of H2SiF2,13 where the Si-F and Si-H equilibrium bond lengths are 157.59(20) and 146.14(20) pm, respectively.13 The theoretical Si-F bond lengths (Tables 1 and 1S) are approximately 2 pm longer than this estimate,13 while the theoretical Si-H bond length agrees to within 0.8 pm, or better. The FsSisF equilibrium angle13 is 107.88(20)°, slightly larger than the theoretical values (same tables). There is also an accurate MW structure of CH3SiHF2.15 The Si-F and Si-H bond lengths in this compound are 158.0(8) and 147.1(10) pm, A B C ∆J ∆JK ∆K δJ δK µa µb µc ∆E 5664.5 1614.2 1400.5 II Rotational Constants 4457.7 1853.1 1647.8 III (MHz) 5703.7 1675.8 1413.9 IV 4044.3 1991.6 1965.2 Quartic Centrifugal Distortion Constantsc (kHz) 0.680 1.82 1.01 0.874 2.34 –0.979 0.479 5.90 6.46 6.31 4.06 –5.97 0.0137 0.130 0.234 –0.028 1.95 2.03 2.96 –62.9 –6.2 –2.8 –0.4 Dipole Moment (10–30 C m) 2.9 –5.4 –2.1 –4.3 6.4 1.9 Energy Differencee (kJ/mol) 0.0 2.7 2.2 7.4 –2.5 0.0d 9.8 a Minima on the potential energy hypersurface; see text. b The MW spectrum of this conformer was assigned. c A-reduction.28 d For symmetry reasons. e Relative to conformer I and corrected for the zero-point energy. Electronic energy of conformer I: -1591 800.51 kJ/mol. respectively, while the F-Si-F angle is 107.1(5)°.15 These experimental values are much closer to their counterparts in H2SiF213 than to the present theoretical results. The quartic centrifugal distortion constants predicted by the two methods vary considerably (Table 2 and 2S). This is not surprising since they depend on the second derivative at the minima of the potential-energy hypersurface. The B3LYP dipole moments (same tables) are generally somewhat smaller than their MP2 counterparts, which is typical. Microwave Spectrum and Assignment of Conformer I. This rotamer is predicted to be the preferred form of the molecule by ∼2-3 kJ/mol relative to the other conformers in both the MP2 and B3LYP calculations (Tables 2 and 2S). I is a prolate asymmetric rotor (Ray’s asymmetry parameter29 κ ∼ -0.90), with µa ≈ 6 × 10-30 C m as its major dipole moment component (Tables 2 and 2S). Pile-ups of aR-branch transitions separated by approximately B + C ∼ 3.01 GHz (Table 2) would therefore be expected to occur in the 40-80 GHz spectral range. A comparatively strong a-type spectrum was not expected despite a sizable µa since as many as seven normal vibrations are calculated to be below 500 cm-1 (Table 4S; Supporting Information). The anharmonic frequencies of the two lowest fundamentals, which are the torsions about the C4-C6 and C6-Si9 bonds, are predicted (same table) to be as low as 56 and 59 cm-1, respectively. This, combined with the fact that the rotational constants are relatively small, means that the partition function is large at -30 °C, rendering a low population in each quantum state and consequently a relatively weak spectrum. Survey spectra revealed several crowded aR pile-up regions separated by about 3.1 GHz, in accord with these predictions. Lines were also observed in the spectral intervals between the pile-ups, but they were much less intense than those found in the pile-ups. Unidentified impurity lines were also encountered. Pairs of aR-lines with identical K-1 g 4 - 6 coalesce, because κ ∼ -0.9. These transitions are modulated at relatively low Stark fields, which facilitated their assignments. The MP2 values of the ∆J and ∆JK centrifugal distortion constants were also Allyldifluorosilane J. Phys. Chem. A, Vol. 114, No. 24, 2010 6611 TABLE 3: Spectroscopic Constantsa,b of the Ground Vibrational State of Conformer I of H2CdCHCH2SiF2H A/MHz B/MHz C/MHz ∆J/kHz ∆JK/kHz ∆K/kHz δJ/kHz δK/kHz ΦJ/Hz ΦJKd/Hz rmse no. transitionsf 5737.3(51) 1592.307(65) 1384.115(66) 0.6555(61) 2.598(15) 6.46c 0.0137c 1.95c 0.0272(48) -0.117(13) 1.510 295 a A-reduction, Ir-representation.28 b Uncertainties represent one standard deviation. c Fixed at this value in the least-squares fit. d Further sextic centrifugal distortion constants preset at zero. e Root-mean-square deviation of a weighted fit. f Number of transitions used in the fit. useful in this respect. No unambiguous assignment could be made for lines with K-1 e 3 because they have slow Stark effects and could not be completely modulated. Overlapping with excited-state lines and spectral weakness were other reasons for not obtaining unambiguous assignments for these transitions. A total of 295 aR-transitions with 9 e J e 26 were ultimately assigned and fitted employing Watson’s A-reduction Irrepresentation Hamiltonian,28 using Sørensen’s program Rotfit.30 b- and c-type lines were searched for, but not found, presumably because the corresponding dipole moment components are much smaller than µa, which is in accord with the calculations (Table 2). The quartic centrifugal distortion constants ∆K, δJ, and δK of this near-prolate rotor could not be determined from the aRlines, and they were therefore held fixed at the MP2 values (Table 2) in the weighted least-squares fit. Inclusion of two sextic constants, ΦJ and ΦJK, were necessary to obtain a satisfactory fit. The spectrum of the ground state is shown in Table 5S in the Supporting Information, and the spectroscopic constants are listed in Table 3. The uncertainties given in Table 5S were used as weights in the least-squares fit. It is seen from Table 3 that an accurate value for the A rotational constant was not obtained from these a-type transitions, whereas the B and C rotational constants have been obtained with high accuracy. Comparison of the theoretical (Table 2) and experimental (Table 3) spectroscopic constants is in order. It is seen from these two tables that the rotational constants of I and III are not very different. The differences between each of the experimental (Table 3) and MP2 rotational constants (Table 2) are less than about 1.4% in the case of I, whereas differences as large as 5.3% are seen for III (the B rotational constant). It has been claimed that MP2 structures are close to equilibrium structures provided a sufficiently large basis set has been used in the calculations.31 This is one evidence that the spectrum shown in Table 5S indeed belongs to I, and not to III. The dipole moment components of the two forms provide additional evidence that I has not been confused with III. µb is calculated to be roughly 80% of the size of µa in III, but only 45% in I (Table 2). The former rotamer should therefore exhibit a relatively much stronger b-type spectrum than I, but no such spectrum was observed. The fact that the two theoretical methods both predict that I is the global minimum is consistent with the experimental findings. Further Conformers. It is seen from Tables 2 and 2S that the remaining conformers II-IV each have at least one dipole moment component of the same order of magnitude as µa of I. Spectra of similar intensities should therefore exist provided they were present with the same concentration as I. This is clearly not the case. Intensity considerations led us to conclude that any rotamer other than I would have been assigned provided its spectrum was roughly one-third as intense as that of I. This makes it possible to estimate that I is at least 2 kJ/mol more stable than any further rotamer. Discussion There are probably several reasons why conformer I is preferred. This global minimum has an ac conformation for the C1sC4sC6sSi9 chain of atoms, just as observed for the preferred forms of H2CdCHCH2SiH318-20 and allyltrifluorosilane H2CdCHCH2SiF3.21 This conformational preference is perhaps a result of repulsion between the silyl or trifluoro silyl groups and the vinyl group in the sp conformation of these two compounds. It is suggested that a similar interaction makes conformers I-III more stable in our case by ∼10 kJ/mol (Table 2 and 2S) compared to IV. There are some structural differences between rotamers I-III that may be responsible for the preference of I over II and III. One of these is the position of H10 (Figure 1) of the difluoro silyl group in I. This atom is closer to the π-electrons of the C1dC4 double bond in this conformer than in II or III. The nonbonded distances between this atom and C1 and C4 atoms are 325 and 366 pm according to the MP2 calculations, compared to the sum, 290 pm, of the van der Waals radii of H, 120 pm, and the half-thickness of an aromatic molecule.32 It is therefore likely that this nonbonded interaction will have a weak stabilizing effect on conformer I compared to II and III, where a similar interaction is not possible. The interaction between the fluorine atoms of the difluoro silyl group and the π-electrons of the vinyl moiety is probably repulsive in nature. Both the very electronegative fluorine atoms are directed away from the π-electrons in I, whereas one fluorine atom is directed toward the double bond in II and III. It is therefore suggested that the weak attraction between the H10 atom and the π-electrons combined with the more favorable orientation of the fluorine atoms in I makes this conformer at least 2 kJ/mol more stable than any other rotamer. Acknowledgment. We thank Anne Horn for her skillful assistance and Alexey Konovalov for recording the spectrum. The Research Council of Norway (Program for Supercomputing) is thanked for a grant of computer time. Also, support from the Summer Undergraduate Research Forum (SURF) at the College of Charleston is gratefully acknowledged. Supporting Information Available: Results of the B3LYP/ 6-311++G(3df,3pd) calculations (bond lengths and angles, rotational constants, distortion constants, dipole moments, vibrational frequencies) and the microwave spectra. This material is available free of charge via the Internet at http:// pubs.acs.org. References and Notes (1) Klaeboe, P.; Nielsen, C. J.; Horn, A.; Guirgis, G. A.; Kilway, K. V. J. Raman Spectrosc. 2009, 40, 2111. (2) Foellmer, M. D.; Murray, J. M.; Serafin, M. M.; Steber, A. L.; Peebles, R. A.; Peebles, S. A.; Eichenberger, J. L.; Guirgis, G. A.; Wurrey, C. J.; Durig, J. R. J. Phys. Chem. A 2009, 113, 6077. (3) Durig, J. R.; Panikar, S. S.; Guirgis, G. A.; Gounev, T. K.; Ward, R. M.; Peebles, R. A.; Peebles, S. A.; Liberatore, R. J.; Bell, S.; Wurrey, C. J. J. Mol. Struct. 2009, 923, 1. (4) Guirgis, G. A.; Panikar, S. S.; El Defrawy, A. M.; Kalasinsky, V. F.; Durig, J. R. J. Mol. Struct. 2009, 922, 93. 6612 J. Phys. Chem. A, Vol. 114, No. 24, 2010 (5) Peebles, S. A.; Serafin, M. M.; Peebles, R. A.; Guirgis, G. A.; Stidham, H. D. J. Phys. Chem. A 2009, 113, 3137. (6) Nashed, Y. E.; Qtaitat, M. A.; Zheng, C.; Zhou, X.; Guirgis, G. A.; Sullivan, J. F.; Durig, J. R. J. Phys. Chem. A 2009, 113, 1653. (7) Guirgis, G. A.; Mazzone, P. M.; Pasko, D. N.; Klaeboe, P.; Horn, A.; Nielsen, C. J. J. Raman Spectrosc. 2007, 38, 1159. (8) Petelenz, B. U.; Shurvell, H. F.; Phibbs, M. K. J. Mol. Struct. 1980, 64, 183. (9) Guirgis, G. A.; El Defrawy, A. M.; Gounev, T. K.; Soliman, M. S.; Durig, J. R. J. Mol. Struct. 2007, 832, 73. (10) Guirgis, G. A.; Horn, A.; Klaeboe, P.; Nielsen, C. J. J. Mol. Struct. 2006, 825, 101. (11) Laurie, V. W. J. Chem. Phys. 1957, 26, 1359. (12) Davis, R. W.; Robiette, A. G.; Gerry, M. C. L. J. Mol. Spectrosc. 1980, 83, 185. (13) D’Eu, J. F.; Demaison, J.; Bürger, H. J. Mol. Spectrosc. 2003, 218, 12. (14) Swalen, J. D.; Stoicheff, B. P. J. Chem. Phys. 1958, 28, 671. (15) Krisher, L. C.; Pierce, L. J. Chem. Phys. 1960, 32, 1619. (16) Møllendal, H.; Guirgis, G. A. Asian Chem. Lett. 2004, 8, 127. (17) Durig, J. R.; Guirgis, G. A.; Zheng, C.; Mohamed, T. A. Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc. 2003, 59A, 2099. (18) Ohno, K.; Taga, K.; Murata, H. Bull. Chem. Soc. Jpn. 1977, 50, 2870. (19) Imachi, M.; Nakagawa, J.; Hayashi, M. J. Mol. Struct. 1983, 102, 403. (20) Guirgis, G. A.; Nashed, Y. E.; Gounev, T. K.; Durig, J. R. Struct. Chem. 1998, 9, 265. (21) Guirgis, G. A.; Nashed, Y. E.; Klaeboe, P.; Aleksa, V.; Durig, J. R. Struct. Chem. 1999, 10, 1. (22) Møllendal, H.; Leonov, A.; de Meijere, A. J. Phys. Chem. A 2005, 109, 6344. Møllendal et al. (23) Møllendal, H.; Cole, G. C.; Guillemin, J.-C. J. Phys. Chem. A 2006, 110, 921. (24) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, revision B.03; Gaussian, Inc.: Pittsburgh PA, 2003. (25) Møller, C.; Plesset, M. S. Phys. ReV. 1934, 46, 618. (26) Becke, A. D. Phys. ReV. A 1988, 38, 3098. (27) Lee, C.; Yang, W.; Parr, R. G. Phys. ReV. B 1988, 37, 785. (28) Watson, J. K. G. Vibrational Spectra and Structure; Elsevier: Amsterdam, 1977; Vol. 6. (29) Ray, B. S. Z. Phys. 1932, 78, 74. (30) Sørensen, G. O. ROTFIT, Personal communication, 1972. (31) Helgaker, T.; Gauss, J.; Jørgensen, P.; Olsen, J. J. Chem. Phys. 1997, 106, 6430. (32) Pauling, L. The Nature of the Chemical Bond; Cornell University Press: Ithaca, NY, 1960. JP101950Z