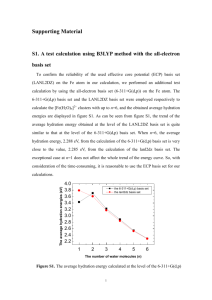
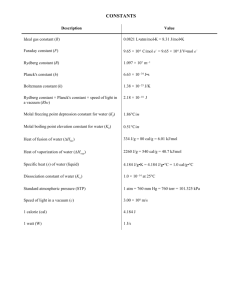
Microwave and Quantum Chemical Study of Propargyl Thiocyanate (HC SC Harald Møllendal,*
advertisement

2300 J. Phys. Chem. A 2010, 114, 2300–2305 Microwave and Quantum Chemical Study of Propargyl Thiocyanate (HCtCCH2SCtN) Harald Møllendal,*,† Alexey Konovalov,† and Jean-Claude Guillemin‡,§ Centre for Theoretical and Computational Chemistry (CTCC), Department of Chemistry, UniVersity of Oslo, P.O. Box 1033 Blindern, NO-0315 Oslo, Norway, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, AVenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France, and UniVersité européenne de Bretagne ReceiVed: December 4, 2009; ReVised Manuscript ReceiVed: January 8, 2010 The MW spectrum of propargyl thiocyanate (HCtCCH2SCtN) has been investigated for the first time in the 25-80 GHz spectral region at room temperature or at 0 °C. The spectra of the ground vibrational state and of the first excited state of the C-S torsional vibration have been assigned for one conformer. This rotamer, denoted ap, has a symmetry plane (Cs symmetry) and an antiperiplanar arrangement for the C-C-S-C link of atoms. It has previously been claimed that a conformer that has a synclinal conformation for this chain of atoms is present in the gas in approximately the same concentration as ap (∼50% of the gas), but this is not supported by the present experiments, where it is shown that the synclinal rotamer, denoted sc, cannot be present in a concentration exceeding 1/3 of the total. It is therefore concluded that ap must be at least 3.0 kJ/mol more stable than sc. The spectroscopic work has been augmented by quantum chemical calculations at advanced B3LYP/aug-cc-pVQZ, B3LYP/6-311++G(3df,3pd), and MP2/aug-cc-pVTZ levels of theory. These theoretical calculations underestimate the energy difference between ap and sc and predict values for the conformationally important C-C-S-C dihedral angle of the hypothetical synclinal form that deviates by ∼10°. Introduction Organic thiocyanates, RSCtN, have a C-S-C angle of ∼100° and may therefore exist in different conformations, owing to restricted rotation about the R-S bond. Relatively few gasphase studies of the conformational properties of thiocyanates have been reported. Ethylthiocyanate (CH3CH2SCN) has been studied by microwave (MW)1 spectroscopy in the gas phase and by infrared (IR) spectroscopy using the matrix-isolation technique.2 The C-C-S-C dihedral angle determines the conformation of this compound. Only the sc conformer, in which this angle is 58° from synperiplanar (sp; 0°), was identified in the MW work.1 The ap rotamer, where the C-C-S-C dihedral angle is 180° from sp, was identified in the matrix-isolation study in addition to the sc form.2 The sc rotamer was determined to be 4.2(3) kJ/mol more stable than the ap conformer.2 Two rotamers, ap and sc, exist for isopropyl thiocyanate (CH3CH(SCN)CH3) according to an IR and Raman study.3 The ap form is the more stable in this case; the enthalpy difference in the gas phase was found to be 2.3(3) kJ/mol using matrixisolation techniques.3 Ethenyl thiocyanate (H2CdCHSCN) has been studied by MW, Raman, and IR spectroscopy.4,5 This compound has a more stable sp form (C-C-S-C dihedral angle ) 0°) and a less stable anticlinal (ac) rotamer, where this angle is ∼130° from sp.4,5 The energy difference is 3-6 kJ/mol in the gas according to the MW study.4,5 Interestingly, a MW study6 of the conformational properties of propa-1,2-dienyl thiocyanate (H2CdCdCHSCN) has shown that they are strikingly different from the properties of the related * To whom correspondence should be addressed. Tel: +47 2285 5674. Fax: +47 2285 5441. E-mail: harald.mollendal@kjemi.uio.no. † University of Oslo. ‡ École Nationale Supérieure de Chimie de Rennes. § Université européenne de Bretagne. ethenyl thiocyanate (H2CdCHSCN). Whereas H2CdCHSCN prefers a sp conformer,4,5 H2CdCdCHSCN prefers an ac form,6 with a C-C-S-C dihedral angle ∼134° from sp. Substitution of a vinyl (H2CdCH-) group with an allenyl group (H2CdCdCH-) therefore has a remarkable effect on the conformational preferences. The examples above demonstrate that the conformational properties of thiocyanates are very sensitive to the group to which the thiocyanate group is attached. It was therefore decided to extend these studies to propargyl thiocyanate (H-CtC-CH2SCtN), where the thiocyanate group is attached to a propargyl (H-CtC-CH2-) moiety. H-CtC-CH2SCtN has previously been investigated by IR and Raman spectroscopy and gas electron diffraction (ED).7 It was found that this compound exists as a mixture of two forms, a less stable C-C-S-C sc form and a more stable ap conformer. The ED rR values for the C-C-S-C dihedral angle were 57(8)° for sc and 171(12)° for ap. The energy difference was determined to be 1.24 kJ/mol with ap as the more stable.7 The methods we have used are MW spectroscopy and highlevel quantum chemical calculations. MW spectroscopy was chosen because of its high accuracy and resolution, making this method especially suitable for conformational studies. The spectroscopic work has been augmented by high-level quantum chemical calculations, which were conducted with the purpose of obtaining information for use in assigning the MW spectrum and investigating properties of the potential-energy hypersurface. Experimental Section Synthesis. The synthesis of this compound has already been reported7,8 and is repeated in the Supporting Information for the convenience of the reader. Microwave Experiment. The spectrum of propargyl thiocyanate was studied in the 25-80 GHz frequency interval by 10.1021/jp9115244 2010 American Chemical Society Published on Web 01/26/2010 Microwave Spectrum of HCtCCH2SCtN Figure 1. Models of the antiperiplanar (ap) and synclinal (sc) conformers of propargyl thiocyanate. The microwave spectrum of ap was assigned. No assignments could be made for sc, which is at least 3.0 kJ/mol less stable than ap. Stark-modulation spectroscopy using the microwave spectrometer of the University of Oslo. Details of the construction and operation of this device have been given elsewhere.9,10 This spectrometer has a resolution of ∼0.5 MHz and measures the frequency of isolated transitions with an estimated accuracy of ∼0.10 MHz. Radio frequency microwave double resonance (RFMWDR) experiments were carried out as described by Wodarczyk and Wilson.11 The compound has a vapor pressure of roughly 80 Pa at room temperature. The experiments were performed at room temperature, or at about 0 °C, with a pressure of roughly 10 Pa. Lower temperatures than 0 °C would have enhanced the intensity of the spectrum, but a study at such low temperatures was not possible owing to insufficient vapor pressure. Quantum Chemical Methods. The present ab initio and density functional theory (DFT) calculations were performed employing the Gaussian03 suite of programs,12 running on the Titan cluster in Oslo. Electron correlation was taken into consideration in the ab initio calculations using Møller-Plesset second-order perturbation calculations (MP2).13 Becke’s three-parameter hybrid functional14 employing the Lee, Yang, and Parr correlation functional (B3LYP)15 was employed in the density functional theory (DFT) calculations. The 6-311++G(3df,3pd) and aug-cc-pVQZ16,17 wave functions were employed in the B3LYP calculations, whereas the aug-cc-pVTZ16,17 wave function was used in the MP2 calculations. The default convergence criteria of Gaussian 03 were observed in all calculations. Results and Discussion Quantum-Chemical Calculations. Rotation about the C4-S7 bond (see Figure 1 for atom numbering) may produce rotational isomerism. The B3LYP/6-311(3df,3pd) electronic energy as a function of the C3-C4-S7-C8 dihedral angle was first calculated in steps of 10° of this dihedral angle using the scan option of the Gaussian03 program. The resulting potential function indicated that there are two minima corresponding to sc and ap, which are depicted in Figure 1. The energies, structures, dipole moments, rotational constants, Watson centrifugal distortion constants,18 and vibration-rotation constants (the R’s)19 were calculated for sc and ap employing the B3LYP/ 6-311(3df,3pd) procedure. Only positive vibrational frequencies J. Phys. Chem. A, Vol. 114, No. 6, 2010 2301 Figure 2. B3LYP/6-311++G(3df,3pd) electronic potential function for rotation about the C4-S7 bond in HCtCCH2SCtN. The values of the C3-C4-S7-C8 dihedral angle in degree are given on the abscissa, and the relative energies in kilojoules per mole are given on the ordinate. ap has a C3-C4-S7-C8 dihedral angle of exactly 180°. A dihedral angle of 69.1° is predicted for sc, which is calculated to be 1.52 kJ/mol less stable than the global minimum, ap. This potential function has maxima at exactly 0° (11.79 kJ/mol above the energy of ap and at 120.0° (6.06 kJ/mol above the energy of ap)). were found in these two cases, which indicate that they are minima (“stable conformers”) on the potential-energy hypersurface. The C3-C4-S7-C8 dihedral angle of ap was found to be exactly 180° from synclinal (0°), resulting in Cs symmetry for this form, whereas this angle was found to be 69.1° in sc. ap was predicted to be 1.50 kJ/mol more stable than sc, after correction for zero-point energies. B3LYP/6-311(3df,3pd) calculations of the energies, geometries, and vibrational frequencies of the two transition states were then performed. The C3-C4-S7-C8 dihedral angle was found to be exactly 0 and 120.0°, respectively for the two transitions states, with energies 11.79 and 6.06 kJ/mol above the energy of ap. Each transition state was found to have one imaginary vibrational frequency, which indicates that they are first-order transition states. The full potential function could now be drawn, as shown in Figure 2. B3LYP calculations with the large aug-cc-pVQZ basis set were also undertaken for ap and sc but limited to the calculations of electronic energies, structures, and dipole moments of the said two forms because these calculations are costly. Interestingly, only relatively small structural differences between the aug-cc-pVQZ structure and the 6-311++G(3df,3pd) structure were seen. The former structure is listed in Table 1, where the ED rR-structure7 is also included for comparison (last column). The bond lengths and bond angles of the ED structure7 are averages of the structures of ap and sc. The B3LYP/aug-ccpVQZ electronic energy difference between sc and ap was predicted to be 1.55 kJ/mol, with ap as the more stable rotamer. Additional results of spectroscopic interest of the two B3LYP calculations are shown in Table 2. The B3LYP/aug-cc-pVQZ results have been listed in this Table when available. B3LYP/ 6-311++G(3df,3pd) harmonic and anharmonic vibrational frequencies as well as the rotation-vibration constants are displayed in Tables S1-S4 of the Supporting Information. MP2 calculations with large basis sets have been shown to predict accurate structures.20 MP2/aug-cc-pVTZ calculations of the structures, dipole moments, vibrational frequencies, and quartic Watson centrifugal distortion constants18 of the two 2302 J. Phys. Chem. A, Vol. 114, No. 6, 2010 Møllendal et al. TABLE 1: MP2/aug-cc-pVTZ and B3LYP/aug-cc-pVQZ Geometries of the ap and sc Conformers of HCtCCH2SCtNa method: conformer: MP2 ap C1-H2 C1-C3 C3-C4 C4-H5 C4-H6 C4-S7 S7-C8 C8-N9 Bond 106.2 121.5 145.0 108.8 108.8 184.2 169.2 117.8 C3-C4-H5 C3-C4-H6 C3-C4-S7 H5-C4-H6 H5-C4-S7 H6-C4-S7 C4-S7-C8 H2-C1-C3 C1-C3-C4 S7-C8-N9 111.6 111.6 107.3 109.9 108.1 108.1 96.9 179.2 178.3 179.7 C3-C4-S7-C8 H5-C4-S7-C8 H6-C4-S7-C8 H2-C1-C4-H5 C1-C3-C4-H5 H5-S7-C8-N9 ap Length (pm) 106.2 106.2 121.5 119.8 144.7 144.6 108.8 108.8 109.1 108.8 183.9 186.5 169.0 169.0 117.8 115.5 Angle (deg) 111.8 111.7 112.4 108.8 108.1 103.6 97.6 179.9 178.0 178.6 Dihedral Angle -180.0 60.5 59.5 -63.4 -59.5 -178.7 118.1 65.4 118.3 74.2 -158.2 162.2 method: EDb B3LYP sc TABLE 2: MP2 and B3LYP Spectroscopic Constants of HCtCCH2SCtNa,b conformer: sc 106.1 119.8 144.4 108.8 109.0 186.0 168.9 115.5 111.6 111.6 108.5 109.5 107.8 107.8 98.8 180.0 179.2 178.4 112.0 111.5 114.1 108.2 107.6 102.8 100.2 179.3 179.4 179.6 (deg) -180.0 59.0 -59.0 -61.5 118.7 -158.5 70.1 -54.9 -169.0 46.1 64.0 171.0 MP2 ap B3LYP sc ap sc b 117.7(4) 142.8(4) 182.7(2) 167.8(2) 115.5(3) 113.7(6) 97.6(9) 57(8) a Entries for the ap conformer, whose MW spectrum was assigned, are in boldface. b Electron-diffraction rR values.7 forms were therefore performed. Selected MP2 results are displayed in Tables 1 and 2. It is noted that these calculations corrected for zero-point vibrations predict sc to be more stable than ap by 0.44 kJ/mol, which is the opposite energy order of what was found in the B3LYP calculations. The theoretical MP2/aug-cc-pVTZ and B3LYP/aug-cc-pVQZ structures in Table 1 should be compared with one another and with relevant experimental structures. Inspection of this Table shows that there are several significant differences in the two theoretical structures. The MP2 triple bonds C1tC3 and C8tN9 are longer than their B3LYP counterparts by ∼2 pm. The equilibrium triple bond in acetylene21 is 120.292(13) pm long. This value is much closer to the B3LYP length (119.8 pm; Table 1) than to the MP2 prediction (121.5 pm) and to the ED rR-result7 (117.7(4) pm; Table 1). The rs CtN triple bond in CH3SCtN is 116.97(20) pm,22 which is somewhat closer to the MP2 result (117.8 pm; Table 1) than to the B3LYP values (115.5 pm) and the ED value7 (115.5(3) pm; Table 1). The experimental C4-S7 bond length (187.7(2) pm) is shorter than the MP2 values by ∼1.5 pm and shorter than the B3LYP result by >3 pm. The MP2 and B3LYP S7-C8 bond lengths agree well but are longer than the corresponding bond in CH3SCtN (168.42(30) pm)22 and the ED result for the title compound.7 The theoretical values of the C4-S7-C8 bond angle differ by up to 2.6° for the individual conformers (Table 1). The corresponding angle is 99.0(1)° in CH3SCtN22 compared with an average ED value of 97.6(9)° in propargyl thiocyanate.7 The ED C3-C4-S7-C8 dihedral angle7 of sc is not very accurate (57(8)°) compared with 60.5 (MP2) and 70.1° (B3LYP/ aug-cc-pVQZ). The MP2 method therefore predicts a much A B C DJ DJK DK d1 d2 Rotational Constants (MHz) 13 864.3 4301.5 14 276.0 1384.0 2339.9 1361.5 1268.5 1646.0 1252.7 Quartic Centrifugal 0.166 -4.37 190.0 ≈0.0 ≈0.0 4756.1 2048.6 1557.1 Distortion Constants (kHz)c 4.58 0.151 3.46 -14.4 -3.98 -18.5 21.5 197.1 42.1 -2.13 -0.0227 -1.52 -0.217 -0.00105 -0.127 Principal-Axes Dipole Moment Components (10-30 C m)d µa 13.9 8.5 13.6 9.7 µb 0.2 10.7 0.1 9.4 µc 0e 0.7 0e 0.8 ∆E (kJ/mol)f 0.40 0.0 0.0 1.55 a Basis set aug-cc-pVTZ has been used in all MP2 calculations. In the B3LYP calculations, the basis set aug-cc-pVQZ has been used to calculate the rotational constants, dipole moment, and energy difference, whereas the 6-311++G(3df,3pd) basis set has been used to calculate the quartic centrifugal distortion constants. Entries for the ap conformer, whose MW spectrum was assigned, are in boldface. c S-reduction Ir-representation.18 d 1 debye ) 3.33564 × 10-30 C m. e For symmetry reasons. f MP2 energy differences have been corrected for zero-point vibrational energies, whereas the B3LYP energies are electronic energies. MP2 energy of sc corrected for zero-point energy: -1591 409.25 kJ/mol. Total electronic B3LYP/aug-cc-pVQZ energy of the ap conformer: -1 594 212.35 kJ/mol. b more compact structure than the B3LYP procedure does for this conformer. Microwave Spectrum and Assignment of ap. The quantum chemical results summarized in Table 2 as well as the previous spectroscopic and ED study7 would imply that both ap and sc would be present in the spectrum, each in relatively large concentration. The dipole moments of these conformations are relatively large, as seen from Table 2. However, a strong MW spectrum was not expected at the temperatures we could employ because the partition functions of the two forms must be relatively large, leaving a low population in each quantum state, primarily because they have several low-frequency vibrational fundamentals. In fact, seven fundamentals of each form are calculated to have frequencies below ∼500 cm-1 (Tables 1S and 3S in the Supporting Information). The fact that the nitrogen atom has a spin of 1 leads to splitting of the rotational transitions by quadrupole interactions. This splitting is not large and unresolved but would lead to broadening of the lines and lower peak intensities. ap should have a relatively strong a-type R-branch spectrum, whereas sc should have strong a- and b-type spectra, according to the principal-axes dipole moments of the two forms. (See Table 2.) Pile-up regions with a complicated fine-structure consisting of aR-transitions of the ground and several vibrationally excited states separated by B + C ≈ 2.6 GHz were expected for ap because this is nearly a prolate rotor (Ray’s asymmetry parameter23 κ ≈ -0.98), whereas several series of b-type Q-branch lines would be the predominating feature of the spectrum of sc. A comparatively weak spectrum was indeed observed in accord with the predictions above. Prominent pile-up regions separated by ∼2.65 GHz were readily observed. These regions are rich in lines originating from the ground and several Microwave Spectrum of HCtCCH2SCtN J. Phys. Chem. A, Vol. 114, No. 6, 2010 2303 TABLE 3: Spectroscopic Constants of the ap Conformer of HCtCCH2SCtNa,b Figure 3. Microwave spectrum of the aR J ) 14 r 13 pile-up region of ap taken at a Stark field of roughly 110 V/cm. g.s. denotes the ground vibrational state, and T1, T2, and T3 indicate successively excited states of the torsion about the C4-S7 bond. The g.s. line, as well as most of the excited-state lines shown here, are actually composed of unresolved transitions with K-1 ) 5-12. The line marked with 4 is the K-1 ) 4 pair of coalescing lines, whereas the two lines marked with 3 are the K-1 ) 3 resolved pair of transitions of the ground state. The pile-up regions become increasingly more complicated than shown in this Figure as J increases because additional resolved splittings of the K-1 states become more pronounced as a result of centrifugal distortion. vibrationally excited states. A typical example is shown in Figure 3. The strongest line of each vibrational state consists of several unresolved pairs of coalescing K-1 lines, with K-1 generally larger than 3. Additional K-1 pairs become resolved from the major peaks as J increases, which is largely a result of the effect of the DJK centrifugal distortion constant.18 The pile-up regions therefore become increasingly complicated as J increases. The theoretical centrifugal distortion constants (Table 2) were very useful for a detailed assignment of the complicated pileup regions because they predict the patterns of the K-1 transitions rather well. A weighted least-squares fit was performed using Sørensen’s program Rotfit.24 Watson’s S-reduction Ir-representation18 was employed in the fitting procedure. Only the DJ and DJK quartic centrifugal distortion constants could be determined. The remaining centrifugal distortion constants DK, d1, and d2 were kept fixed at the B3LYP/6-311++G(3df,3pd) values (Table 2) in the least-squares fit. Isolated lines were weighted according to the experimental uncertainty ((0.10 MHz). Several K-1 lines of each J + 1 r J transition overlap and have therefore been assigned much larger weights, as shown in Table 5S in the Supporting Information, where the groundstate spectrum is listed. Attempts to find b-type transitions failed, presumably because µb is almost negligible (Table 2). The spectroscopic constants of the ground state are displayed in Table 3, where it is seen that the pseudo-inertial defect ∆ ) Ic - Ia - Ib ) -3.15(1) × 10-20 u m2. This value is typical for compounds having a symmetry plane and two out-of-plane sp3hybridized hydrogen atoms. The ∆ values calculated from the rotational constants in Table 2, using a conversion factor of 505 379.05 × 10-20 MHz u m2, are -3.20 (MP2) and -3.15 × 10-20 u m2 (B3LYP), respectively, which is very similar to ∆ given in Table 3. The rotational constants of the ground vibrational state (Table 3) should be compared with their counterparts in Table 2. vib. state groundc first excited torsional stated A/MHz B/MHz C/MHz ∆/10-20 u m2f DJ/kHz DJK/kHz DK/kHzg d1/kHz d2/kHz rmsh no. trans.i 14 134.0(39) 1382.5018(79) 1269.3008(79) -3.15(1) 0.16763(87) -4.356(17) 197 -0.0227 -0.00105 1.099 233 13 715e 1383.5566(95) 1272.3385(98) -4.919(5) 0.1768(15) -4.348(38) 197 -0.0227 -0.00105 1.199 96 a Uncertainties represent one standard deviation. b S-reduction Ir-representation.18 c Spectrum listed in Table 5S in the Supporting Information. d Fixed, see text. e Spectrum shown in Table 6S in the Supporting Information. f Conversion factor 505379.05 × 10-20 MHz u m2. ∆ ) Ic s Ia s Ib. g DK, d1, and d2 were fixed in the least-squares fit. See the text. h Root-mean-square deviation of a weighted least-squares fit. See the text. i Number of transitions used in the fit. Complete agreement cannot be expected because the effective constants of Table 3 are defined differently from the theoretical constants displayed in Table 2. It is seen that the experimental constants agree to within 2%, or better, with both the MP2 and the B3LYP results. This is a satisfactory result taking into account the different definitions of the rotational constants. It is therefore not possible to conclude from a comparison of the rotational constants which theoretical method predicts the more accurate structure. It is also seen that the B3LYP and experimental DJ and DJK centrifugal distortion constants agree well. Vibrationally Excited States. The ground vibrational state a R-branch transitions were accompanied by several vibrationally excited states. (See Figure 3.) The most prominent series is the first excited state of the torsion about C4-S7 bond, denoted T1 in this Figure, which has a frequency of 55 cm-1 according to the B3LYP calculations (Table 1S in the Supporting Information). Unambiguous assignments of the K-1-fine structure transitions of the excited-state transitions were very difficult to obtain because of frequent overlaps and comparatively low intensities. However, it was possible to assign several lines with K-1 g 2 of T1. This was not sufficient to obtain an accurate value of the A rotational constant because of the near-prolacy of ap. The A rotational constant was therefore fixed at 13 715.0 MHz in the least-squares fit. This value was derived using the vibration-rotation constant RA ) 419.0 MHz, as given in Table 2S in the Supporting Information. RA is defined by RA ) A0 A1,19 where A0 is the rotational constant of the ground state and A1 is the corresponding constant of the first excited state of a particular vibrational mode. The spectrum of the T1 state is listed in Table 6S in the Supporting Information, whereas the spectroscopic constants are listed in Table 3. The vibration-rotation constants associated with the B and C rotational constants, RB and RC are defined in a manner analogous to RA.19 The experimental value obtained from the rotational constants of Table 3 are RB ) -1.055(12) and RC ) -3.038(13) MHz, in satisfactory agreement with the B3LYP values of 0.03, and -2.84 MHz, respectively (Table 1S in the Supporting Information). Searches for sc. The numerous absorption lines seen outside the pile-up regions of ap are all relatively weak, which is one indication that there is not a high concentration of sc because this conformer should have relatively large µa and µb dipole 2304 J. Phys. Chem. A, Vol. 114, No. 6, 2010 moment components (Table 2) and a statistical weight of 2 relative to the weight of ap, which is 1 because there are two mirror-image forms of sc. Studies of the spectrum at comparatively low Stark field strengths (∼100 V/cm) simplify the spectrum significantly because a majority of the transitions are not modulated under these conditions, and this was exploited in an attempt to assign sc. However, the coalescing and near-coalescing pairs of the highest K-1 aR-lines of both ap and sc should be completely modulated at this field strength. Their intensities depend critically on µa2, as well as on the statistical weight of each conformer. µa of ap is larger than µa of sc (Table 2), but the fact that latter form has a statistical weight of 2 compared with 1 for ap means that the intensities of these aR-lines would be comparable if both forms were present in a concentration of 50% each, as claimed in the previous study.7 Many attempts to find the said aR-lines transitions of sc failed, and there are no relatively strong unassigned transitions in the regions predicted for these lines, which is another strong indication that the gas composition is predominated by ap. The RFMWDR technique11 was also employed in an attempt to find selected aR-lines of sc, however, with negative results. It is concluded that the spectrum of sc would have been assigned provided that the concentration of this form were larger than ∼1/2 of the concentration of ap. Assumption of a Boltzmann distribution of the energy and a statistical weight of 2 for sc therefore leads to the conclusion that ap is at least 3.0 kJ/mol more stable than sc. This result, which is considered to be conservative, is at variance with the previous experimental result, which reported an energy difference of only 1.24 kJ/mol, with ap as the more stable form.7 Interestingly, the theoretical energy calculations (Table 2) underestimate the energy difference by more than 3.5 (MP2) and 1.6 kJ/mol (B3LYP). Conclusions The MW spectrum of HCtCCH2SCtN has been investigated for the first time. The spectra of the ground vibrational state and of the first excited state of the C-S torsional vibration have been assigned for one conformer denoted ap, which has a symmetry plane (Cs symmetry) and an antiperiplanar arrangement for the C-C-S-C link of atoms. A previous ED and vibrational spectroscopic study7 claims that a conformer that has a sc conformation for this chain of atoms is present in the gas in approximately the same concentration as ap (∼50% of the gas). Assuming the statistical weight of sc to be 2, compared with 1 for ap, the energy difference was estimated to be 1.2 kJ/mol, with ap as the more stable.7 Extensive searches have been undertaken in an attempt to assign the spectrum of sc. However, intensity considerations, Starkmodulation patterns, and RFMWDR searches indicate that if sc is present, then it has to be in a concentration significantly <50%. In fact, sc cannot be present in a concentration exceeding 1/3 of the total. ap must therefore be at least 3.0 kJ/mol more stable than sc. The spectroscopic work has been augmented by quantum chemical calculations at B3LYP/aug-cc-pVQZ, B3LYP/6311++G(3df,3pd), and MP2/aug-cc-pVTZ levels of theory. The B3LYP calculations predict that ap is more stable than sc by ∼1.5 kJ/mol, whereas the MP2 calculations indicate the opposite, namely, that sc is the more stable form by 0.44 kJ/ mol. These theoretical calculations therefore underestimate the energy difference between these rotamers. However, spectroscopic constants of ap are well predicted in both the MP2 and B3LYP calculations. It is therefore not possible to decide which Møllendal et al. method predicts the more accurate structure of ap from a comparison of the experimental and theoretical rotational constants. Interestingly, a significant difference is predicted in the theoretical structures of the hypothetical sc form. The conformationally important C-C-S-C dihedral angle of the hypothetical sc form is calculated to be 60.5° in the MP2 calculation, ∼10° less than the B3LYP/aug-cc-pVQZ prediction (70.1°). It is concluded that the theoretical calculations even at the high levels of theory reported herein are still insufficient to predict correct energy difference between the two forms and a consistent structure for sc. The present conformational findings for HCtCCH2SCtN resemble the results for the selenium analogue, HCtCCH2SeCtN, where a conformation similar to ap was identified in the MW spectrum and is presumed to be the more stable form of the molecule.25 No similar experimental information appears to be available for the oxygen member (HCtCCH2OCtN) of this series of cyanates. Acknowledgment. We thank Anne Horn for her skillful assistance. The Research Council of Norway (Program for Supercomputing) is thanked for a grant of computer time. A.K. thanks The Research Council of Norway for financial assistance through contract 177540/V30. J.-C.G. thanks the PID OPV (CNRS) for financial support. Supporting Information Available: The synthesis, results of the B3LYP/6-311++G(3df,3pd) calculations and the microwave spectra. This material is available free of charge via the Internet at http://pubs.acs.org. References and Notes (1) Bjørseth, A.; Marstokk, K.-M. J. Mol. Struct. 1972, 11, 15. (2) Braathen, G. O.; Gatial, A.Spectrochim. Acta, Part A 1986, 42A, 615. (3) Jozwiak, R. N.; Karlsson, A.; Klaeboe, P.; Nielsen, C. J. J. Raman Spectrosc. 1994, 25, 571. (4) Beukes, J. A.; Klaeboe, P.; Møllendal, H.; Nielsen, C. J. J. Raman Spectrosc. 1995, 26, 799. (5) Beukes, J. A.; Klaeboe, P.; Møllendal, H.; Nielsen, C. J. J. Mol. Struct. 1995, 349, 37. (6) Møllendal, H.; Cole, G. C.; Guillemin, J.-C. J. Phys. Chem. A 2007, 111, 2542. (7) Midtgaard, T.; Gundersen, G.; Nielsen, C. J. J. Mol. Struct. 1988, 176, 159. (8) Yura, Y. Chem. Pharm. Bull. 1962, 10, 1094. (9) Møllendal, H.; Leonov, A.; de Meijere, A. J. Phys. Chem. A 2005, 109, 6344. (10) Møllendal, H.; Cole, G. C.; Guillemin, J.-C. J. Phys. Chem. A 2006, 110, 921. (11) Wodarczyk, F. J.; Wilson, E. B., Jr.J. Mol. Spectrosc. 1971, 37, 445. (12) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, revision B.03; Gaussian, Inc.: Pittsburgh, PA, 2003. (13) Møller, C.; Plesset, M. S. Phys. ReV. 1934, 46, 618. (14) Becke, A. D. Phys. ReV. A 1988, 38, 3098. (15) Lee, C.; Yang, W.; Parr, R. G. Phys. ReV. B 1988, 37, 785. (16) Dunning, T. H., Jr. J. Chem. Phys. 1989, 90, 1007. (17) Peterson, K. A.; Dunning, T. H., Jr. J. Chem. Phys. 2002, 117, 10548. Microwave Spectrum of HCtCCH2SCtN (18) Watson, J. K. G. Vibrational Spectra and Structure; Elsevier: Amsterdam, 1977; Vol. 6. (19) Gordy, W.; Cook, R. L. MicrowaVe Molecular Spectra. In Techniques of Chemistry; John Wiley & Sons: New York, 1984; Vol. XVII. (20) Helgaker, T.; Gauss, J.; Jørgensen, P.; Olsen, J. J. Chem. Phys. 1997, 106, 6430. (21) Herman, M.; Campargue, A.; El Idrissi, M. I.; Vander Auwera, J. J. Phys. Chem. Ref. Data 2003, 32, 921. J. Phys. Chem. A, Vol. 114, No. 6, 2010 2305 (22) Dreizler, H.; Rudolph, H. D.; Schleser, H. Z. Naturforsch., A 1970, 25, 1643. (23) Ray, B. S. Z. Phys. 1932, 78, 74. (24) Sørensen, G. O. J. Mol. Spectrosc. 1967, 22, 325. (25) Møllendal, H.; Mokso, R.; Guillemin, J.-C. J. Phys. Chem. 2009, 113, 2821. JP9115244