The Iterative Adaptive Approach in Medical Ultrasound Imaging Member, IEEE
advertisement

1
The Iterative Adaptive Approach in Medical
Ultrasound Imaging
Are Charles Jensen, Member, IEEE, and Andreas Austeng, Member, IEEE
(Note: This is a draft.)
Abstract—Many medical ultrasound imaging systems are based
on sweeping the image plane with a set of narrow beams. Usually,
the returning echo from each of these beams is used to form
one or a few azimuthal image samples. We model, for each
radial distance, jointly the full azimuthal scanline. The model
consists of the amplitudes of a set of densely placed potential
reflectors (or scatterers), cf. sparse signal representation. To fit
the model, we apply the iterative adaptive approach (IAA) on
data formed by a sequenced time delay and phase shift. The
performance of the IAA in combination with our time delayed
and phase-shifted data is studied on both Field II simulated
data of scenes consisting of point targets and hollow cystlike structures, and recorded ultrasound phantom data from
a specially adapted commercial scanner. The results show that
the proposed IAA is more capable of resolving point targets
and gives better defined and more geometrically correct cyst-like
structures in speckle images compared to the conventional delayand-sum (DAS) approach. Compared to a Capon beamformer, the
IAA showed an improved rendering of cyst-like structures and a
similar point-target resolvability. Unlike the Capon beamformer,
the IAA has no user parameters and seems unaffected by signal
cancellation. The disadvantage of the IAA is a high computational
load.
I. I NTRODUCTION
WO-DIMENSIONAL medical ultrasound images are often formed using a one-dimensional (1D) linear array
of transducers where each element in the array is capable of
both transmitting and receiving acoustic energy [1]. Pulses are
transmitted into the organ of interest, causing backscatter to
form at interfaces between materials with different acoustic
impedance. The array records the backscatter and processes it
to form image samples. The conventional way to form image
samples is known as delay-and-sum (DAS) beamforming [2],
which, for a given sample location, adds time delays to
the channels to align the returned echo before summing or
averaging the outputs.
Most systems use focused transmit beams, i.e., the insonified spatial coverage of each transmitted beam is limited to a
narrow sector. A full image is then formed by sweeping the
scene with a set of such beams. The presented work is based
upon this narrow transmit-beam approach.
Adaptive beamformers (BFs) have been promoted as an
alternative to the DAS BF in medical ultrasound imaging,
and significant improvements have been reported [3]–[11],
although they have currently not been demonstrated in clinical
settings. These improvements come at a cost of an increased
computational load and the introduction of additional user
parameters. The parameters control the degree of adaptivity
of the BFs, and the obtained performance for a given set
T
of parameters depends on the imaged scene and how much
interference and noise there is in the recorded data. This
dependence on user parameters limits the adoption of adaptive
approaches to beamforming.
Recently, a promising parameter-free, sparse modelling approach, termed iterative adaptive approach (IAA) [12]–[14]
has been introduced as an alternative adaptive BF. The IAA
is based on modelling a dense set of potential narrowband
signals impinging on the array. Such approaches are often
termed, or linked to terms, like sparse modelling or sparse
signal representation, since in many applications, e.g. source
localisation, the potential, or modelled, sources greatly outnumber the actual sources. In our setting, the IAA will be
modelling jointly the amplitude of all the potential reflectors
(or scatterers) for a given radial distance. However, to be able
to apply such an approach in our multi-beam, pulsed data
setting, we have to transform the data so that it can be the
basis for the phase-based (narrowband) model. We achieve
this by a two-step process in which we first align the data
through time delays, a step identical to that of the DAS BF,
then do a per-element phase shift. This two-step process is
also at the core of the multi-beam Capon BF1 introduced in
[11]; here, however, we use it as a basis for applying the IAA.
To evaluate the proposed algorithm, we perform experiments on both Field II [15], [16] simulated data of scenes
consisting of point targets and hollow cyst-like structures, and
recorded ultrasound phantom data from a specially adapted GE
Vivid E9 scanner. The resulting images are compared to those
of a conventional DAS BF, and a (non-iterative) Capon BF.
We start by describing the studied imaging setup and how
we form images based on the DAS BF in Section II, before
introducing the IAA and our take on adapting it to our imaging
setting in Section III. Sections IV and V detail the experiments
and report on their results. A discussion and concluding
remarks can be found in Section VI.
II. BACKGROUND
Figure 1 illustrates the imaging setup. By adjusting the
timing of the emission for each element in our M -element
array, we transmit a series of K steered and focused beams.
The returning signal is recorded using the same array. To
form a particular DAS BF image sample, the M recorded
array signals are time delayed so that the backscatter from the
location of the sample is summed up coherently, i.e., we use
1 The Capon BF is also known as the minimum-variance BF or the
minimum-variance distortionless-response BF [17].
2
from such a single reflector becomes:
E x(t)x(t)T = |s|2 aθ aTθ ,
2
(3)
2
where |s| = E[|s(t)| ]. In the case of multiple uncorrelated
reflectors, we get a covariance matrix that is the sum of such
matrices. Now, let our model consist of K̄ such reflectors
spread densely across the azimuthal scanning field. The modelled covariance matrix then becomes:
R̄ =
Figure 1. The studied imaging setup consists of firing sequentially a set of
narrow beams. The backscatter from each beam form image samples along
its path. Illustration from [11].
time delays to focus at the sample point. We will for simplicity
assume that we create a single radial line of image samples
from the backscatter of each transmitted beam.
Let xk,n be a column-vector containing the M sampled
complex-valued signals for such a time-delayed array when
recording from an angle θk , 1 ≥ k ≥ K, at range-sample
n. Using this notation, the non-gain-compensated DAS BF
image-sample along the kth beam at range-sample n is:
1 T
1 xk,n ,
(1)
M
where 1 is a length M column-vector of ones and the
superscript T denotes the conjugate transpose. To form the
image samples along an entire azimuthal scanline, cf. that
which will be jointly modelled in the IAA, (1) is calculated
with a fixed n and with k varying between 1 and K.
Reduced sidelobe leakage at the expense of a wider resolution cell can be achieved by replacing the uniform weights
by tapered ones; we, however, will stick to this uniformlyweighted version in our experiments.
A. Azimuthal scanline modelling and the IAA algorithm
The IAA is in our setting based on explicitly modelling
reflectors along an azimuthal scanline of the image plane. A
necessary assumption is that of a narrowband signal waveform.
We will later see how we can transform our pulse-based data
to fit this approximately. Now, if we have a reflector at a range
n and angle θ, the (non-focused) received signal at the array
can be modelled as:
x(t) = s(t)aθ,n ,
where s(t) is the signal waveform, and aθ,n is a steering
vector which introduces phase-shifts based on the distances
from the reflector to the array elements. In the experiments
we have applied a plane-wave approximation, as argued for in
[11]. This means that we can ignore n and get the following
simplified expression:
h
iT
aθ = 1 ejπ sin(θ) ej2π sin(θ) . . . ej(M −1)π sin(θ) , (2)
where we have assumed a linear array with a one-half wavelength pitch. The resulting M × M array covariance matrix
|sk |2 aθk aTθk = APAT ,
(4)
k=1
where A is a M × K̄ matrix of steering vectors and P is a
diagonal matrix with the squared amplitudes, |sk |2 , along its
diagonal. Note that with the plane-wave approximation in (2),
the model is identical to that used in the original line-spectrum
estimation application of IAA.
Assume now that we have some array samples which are
used to form a sample covariance matrix; R̂. An initial
estimate of the power of each reflector can be made by
applying matched spatial filtering:
T
P̂init
kk = aθk R̂aθk .
(5)
This corresponds to a narrowband version of the (although
square-valued) DAS BF. The IAA algorithm consists of starting with this estimate of P, and then iterating the following
three steps:
1) The current amplitude-squared estimate matrix, P̂, is
used in (4) to form the model-based covariance matrix estimate, R̄.
2) A set of weights is formed by solving the minimumvariance distortionless-response criterion (cf. the Capon BF)
for each potential reflector:
min wkT R̄wk ,
wk
III. I TERATIVE ADAPTIVE APPROACH
K̄
X
s.t. wkT aθk = 1
⇓
R̄−1 aθ
wk = T −1 k .
aθk R̄ aθk
(6)
3) These weights are in turn used to form a new set of
estimates for the squared reflector amplitudes:
P̂kk = wkT R̂wk .
(7)
In the end, the algorithm converges and the diagonal of P̂
contains the amplitude squared of each potential reflector. Note
that in addition to this model-restricted, iterated Capon explanation, one can also interpret IAA as fitting the model R̄ to
the data R̂ by maximizing an approximation to the likelihood
based on an assumption of normally distributed, circularly
symmetric, zero-mean data. Please see the references for IAA
for further details of the algorithm.
B. Pulsed system, and multiple beams
The challenge when applying the IAA in our pulse-based
imaging system is that we cannot form viable sample covariance matrices directly. That is, we cannot simply add together
array outer-products from echoes arriving from different angles
3
when forming the per-range covariance matrices. To overcome
this problem, we apply the sequenced time delay and phasebased focusing approach described in [11]. In that paper we
built multi-beam covariance matrices by utilizing that the
imaging system transmitted focused beams, and that within
the sector that each beam illuminated, a phase-based steeringvector approximation was valid after employing a time delay.
The time-delayed and phase-shifted data was then the basis
for an outer-product sample covariance matrix.
More precisely, let xk,n again contain the sampled array
when focusing (using time delay) at range n and angle θk ,
and let Xn = [x1,n x2,n . . . xK,n ]. Now, let A be an M × K
steering matrix in which every column is a steering vector
pointing (through phase shifts) in turn towards each of those
locations:
(8)
A = [aθ1 aθ2 . . . aθK ] .
The phase-based (narrowband) approximation to the impinging
signals from range n can now be found by multiplying every
time-delayed array sample by the corresponding phase-based
steering-vector sample:
X̃n = A ◦ Xn ,
(9)
where ◦ denotes the Hadamard (point-wise) product. The outer
products of these phase-steered array samples can then be
averaged to form a covariance matrix estimate:
R̂n =
1 T
X̃ X̃n .
K n
(10)
The R̂n can now be applied in the IAA algorithm when
updating the modelled reflector-amplitude estimates, cf. (7):
P̂kk = wkT R̂n wk .
(MB)
(11)
However, if we assume that the number of array samples is
equal to the number of modelled reflectors, i.e., K̄ = K and
that the steering vectors in (8) are equal to that of (4), we can
also update the squared amplitude estimate of each reflector
by:
2
Pkk = wkT x̃k,n ,
(SB)
(12)
where x̃k is the kth column of (9), i.e., we use the samples
from a single beam when estimating the amplitude from that
specific location. We will refer to this latter approach as
applying single-beam (SB) power in the iterations, and when
we apply the full R̂n , we will refer to it as applying multibeam (MB) power. It should be noted, however, that one needs
to employ a beamspace projection as discussed in Section III-C
to be able to use the SB in the iterations, since typically the
actual imaged sector does not span the full image plane.
When the IAA algorithm has converged, we have from (6)
a set of array weights for every modeled reflector location.
As in the iteration step, we can choose to apply these weights
to the full covariance matrix when estimating the final pixel
values (MB). Or, if we have matching modelled and received
steering matrices, cf. (4) and (8), we can choose to apply
the weights to the corresponding phase-shifted array sample
(SB). This leaves us with four immediate versions of the
IAA; either use MB or SB in the iterations for finding the
weights, and similarly choose between MB and SB when
Figure 2. A block diagram showing the steps of the proposed algorithm. Note
the two-step pre-processing consisting of time-delay focusing and phase-based
steering.
forming the final pixel values. We will refer to these four
combinations as IAA (MB/SB), IAA (MB/MB), IAA (SB/SB)
and IAA (SB/MB), where the first two letters in parenthesis
indicate the approach used in the IAA iterations and the latter
letters whether (11) or (12) has been applied to form the final
pixel values. Applying MB to form the final pixel values will in
effect compound the full images from each transmitted beam
incoherently. Such compounding is often done to reduce noise
or speckle. A summarizing depiction of the processing steps
is found in Figure 2.
An obvious extension to the SB and MB, both in the IAA
iterations and when forming the final pixel values, would be
to weight the contributions from the different beams in such
a way as to emphasize the beams closest to that of the output
image sample. This will of course come at a cost of additional
parameters. We leave this as a possible future study.
C. Beamspace projection
Typically we image only a certain sector of the image plane,
and hence the placement of the modelled reflectors should
also be limited to lie within this sector. However, for the
R̄ to be invertible, we have to model the full ±90◦ angular
span, add a diagonal loading factor, or linearly transform the
data and the model onto a reduced-dimensional beamspace
[17]. We opt for the latter, as it yields faster algorithms and
requires no additional user parameters. A simple approach to
beamspace transformation is to let the transformation matrix
be the Fourier transform basis that spans the lower spatial
frequencies that cover the actually imaged sector. If we let
Bbs be the M × N linear transform matrix transforming from
M to N < M dimensions, we get:
R̄bs = Abs PATbs ,
Abs = BTbs A,
and also a reduced-sized sample covariance matrix:
T
R̂bs
n = Bbs R̂n Bbs .
(13)
This way the modelled reflectors do not need to span the full
±90◦ degrees and are hence reduced in number. Furthermore,
the reduction in dimensionality of the covariance matrix yields
computational benefits, as its inverse needs to be calculated in
every iteration.
D. The multi-beam Capon algorithm
A closely related BF is the Capon BF, which finds noniteratively the weights that minimize the same criterion as in
(6), although with the model covariance matrix, R̄, replaced
4
by the sample covariance matrix R̂. However, as described in
[11], the approach requires in practice a parameter, δ > 0,
guiding a diagonal loading of R̂ before the matrix inverse is
calculated. That is, the R̄−1 in (6) is replaced by:
−1
1
R̂−1
.
(14)
n = R̂n + δ M tr{R̂n }I
This diagonal loading ensures numerical stability, reduces possible signal cancellation, and effectively increases the tolerance
of focusing mismatches.
The adaptive weights can then be used to create image samples in the same manner as for IAA, i.e., we have immediately
both an SB and MB version, denoted (-/SB) and (-/MB) since
there are no iterations in the Capon BF.
IV. E XPERIMENTS
To evaluate the suggested IAA approach to beamforming,
we have run the algorithms on simulated data from scenes
of point-targets and scenes of cysts in speckle, in addition to
recorded ultrasound phantom data. The results are compared
to the output of a conventional, uniformly-weighted, DAS BF
and the Capon BF described in Section III-D. The algorithms
were implemented in MATLAB (Mathworks, Inc.), and the
matrix operations, including matrix inversion, were done using
standard, built-in functions.
To reveal imaging details of high-resolution adaptive BFs,
a high image sample density is needed. In the experiments we
achieve this by transmitting a rather high number of beams.
An alternative is to perform upsampling at reception, see e.g.
[18]. Even though such an approach would yield an increased
frame rate, it would entail a choice on the actually applied
technique and possibly introduce imaging artifacts. However,
the phantom data has also been processed using fewer beams
to validate that the suggested IAA approach is capable of
directly handling more typical imaging scenarios.
A. Simulated point-reflector and cyst data
The imaging setup for the Field II simulations consisted of
a 96-element array with a one-half wavelength pitch, a center
frequency of 3.5 MHz and a relative bandwidth of 96%. A
three-period sine was used as excitation, and the transmit focus
was set to 8 cm. K = 485 transmit and receive beams were
distributed within ±30◦ .
In order to study the upper resolution capabilities of the
different BFs, we simulated data from a scene consisting of
two points located perpendicular to the array at an angle θ
apart at a depth of 8 cm. The angular separation of the two
targets were gradually varied from 0◦ to 3◦ . For every angular
separation of the point targets, 33 speckle realizations were
available.
In the simulated scenes containing cysts, four cysts were
created by removing reflectors within cylinders of diameters
one and two cm. Again we simulated 33 speckle realizations.
For the Capon BF we applied the same diagonal loading
factors as suggested in [11], δ = 0.5 when creating SB
outputs and δ = 0.01 when creating MB outputs. As in [11],
we had linear arrays, and hence employed forward-backward
averaging. For both the Capon BF and IAA, a beamspace
transform down to 49 dimensions was applied. This beamspace
captures close to all of the variance for incoming, narrowband,
far-field signals arriving from ±30◦ .
To demonstrate the IAA’s robustness to signal cancellation,
the point-reflector data were also simulated using a setup
which transmitted using only the central half of the 96-element
array (Half Tx), i.e., the system used wider transmit beams.
B. Recorded phantom data
A specially adapted GE Vivid E9 scanner with a 96-element
1D phased array probe was used to scan a tissue-mimicking
Gammex 403GS LE phantom. The center frequency of the
transmitted pulse was 3.5 MHz and the beams had a focus at
a depth of 8 cm, mirroring that of the simulated scenes. A total
of K = 432 transmit and receive beams were swept within
±37.5◦ , a slightly wider sweep than in the simulated setup.
Unfortunately, the outer elements of the array seemed to be
faulty, hence we ended up with using the 94 central elements
at reception.
The parameters for the Capon BF were the same as for the
simulated scenes. The beamspace dimension, however, was
increased to 59 to account for the wider azimuthal sweep.
The output images of the BFs were scaled to make them
have a mean background value of zero dB.
In a clinical imaging setting based on the DAS BF, the
azimuthal sample rate is typically at least that of the Nyquist
sample rate of the combined transmit and receive beampattern.
In our setting that would mean we would have at least
Naz = 2 sin(37.5◦ )/ sin(λ/(Dtx + Drx )) ≈ 117 azimuthal
samples, or beams, where Dtx and Drx are the array transmit
and receive aperture, respectively. An easily attained increase
of samples, or beams, would be to upsample at reception by a
factor two. This gives us about the same number of beams as
if we would use only every second in the recorded phantom
data set. Processing this data is close to processing that which
stems directly from a typical imaging system.
C. Image quality assessment
We report two common image quality metrics: 1) the
speckle signal-to-noise ratio and 2) the contrast-to-noise ratio. The speckle signal-to-noise ratio is defined as the ratio
between the produced image amplitude mean value µ and its
standard deviation σ in homogeneous regions:
SNR = µ/σ.
(15)
The applied contrast-to-noise ratio, CNR, for a region-ofinterest (ROI) in the dB-scaled images with a scattering level
different from the background, is
|µROI − µB |
,
CNR = p 2
σROI + σB2
(16)
where µROI and µB are the mean intensity in the ROI and
background, respectively, and σROI and σB are the corresponding standard deviations. An outline of the ROI used
for the calculations in the simulated data is superimposed on
the example DAS rendering in Section V. The ROI for the
5
V. R ESULTS
−10
A. Point reflectors
B. Simulated speckle data
Processed cyst-scene data can be found in Figure 5. From
the images we can see that both the Capon BF (-/SB) and
the IAA (MB/SB) give images that are quite similar to the
conventional DAS BF, although the size of the cysts are a bit
more narrow for the DAS BF. The (MB/MB) output, however,
shows a clear smearing of the speckle, an effect that was also
seen in the point-target experiments. Even though the speckle
has been smoothed, the cysts are well defined. The reduction
in speckle variance can be quantified by the SNR in (15). From
Table I we can clearly see that the (xx/MB) approach acts as
a speckle reduction technique, as the corresponding SNRs are
significantly higher. Even though the apparent dynamic range
−20
−30
−40
−50
−10
−5
0
5
10
15
20
3
4
Azimuthal angle (deg)
Amplitude (dB)
0
−5
−10
−15
−20
−2
−1
0
1
2
Azimuthal angle (deg)
(a) Full Tx
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
0
Amplitude (dB)
−10
−20
−30
−40
−50
−10
−5
0
5
10
15
20
3
4
Azimuthal angle (deg)
0
Amplitude (dB)
Examples of azimuthal scanlines covering the two point
reflectors can be found in Figure 3. A value of zero dB
corresponds to the mean output from the BF when focusing on
a single point-target. All the adaptive BFs are able to separate
the two point-reflectors, although there is a sharper divide
created by the Capon BF than that created by the IAA. We
also see that the IAAs using SB in the iterations (SB/xx),
show a sharper divide than the IAAs applying MB (MB/xx).
When illuminating the scene with a wider transmit beam, the
Capon BF shows signs of signal cancellation, as there is a
reduced apparent amplitude at the locations of the reflectors.
In both the wide and narrow transmit-beam case, applying
MB when producing the final pixels gives much smoother
azimuthal scanlines, although at the cost of a reduced dip
between the point-reflectors.
Figure 4 gives a further indication of the resolution capabilities of the different approaches. For a gradually increased
distance between the point targets, Figure 4 shows the ratio
between the minimum amplitude between the two peaks and
that of the peaks themselves based on the average of 33 speckle
realizations. The horizontal dashed line in the figure is at a
ratio of 0.5, or −6 dB, a ratio that could arguably be used
as a threshold for resolvability of the two point targets. The
curves are quite stable in the sense that statistical bootstrapping
indicates a standard deviation around this 0.5 ratio to be less
than 0.015 degrees for all approaches. From the figure we see
that all the adaptive BFs have better point-resolving power than
the DAS BF. The Capon BF (-/SB) and the IAA (SB/SB) do
about equally well, while the IAA (MB/SB) is doing slightly
worse. For both the IAA and the Capon BF, applying MB
for the final pixel value reduces the ability to resolve the two
reflectors when transmitting narrow beams (Full Tx). When
transmitting wider beams (Half Tx), it is the other way around
for the Capon BF.
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
0
Amplitude (dB)
phantom data is similarly shaped and placed at the location of
the cyst.
The reported standard-deviation measures of these values
for the simulated scenes stem from statistical bootstrapping,
i.e., we created new data sets by random-sampling with
replacement the speckle realizations.
−5
−10
−15
−20
−2
−1
0
1
2
Azimuthal angle (deg)
(b) Half Tx
Figure 3. Example of azimuthal scanlines from scenes where the two point
reflectors are not resolvable using the DAS BF. The bottom plots are “up
close” versions of the ones above. a) and b) show, respectively, imaging setups
using narrow and wide transmit beams. The targets are separated by about 1
degree for a) and about 1.3 degrees for b).
6
25 dB
0.04
0.05
25 dB
0.04
0.05
15 dB
0.06
15 dB
0.06
5 dB
0.07
0.08
5 dB
0.07
0.08
−5 dB
0.09
−5 dB
0.09
−15 dB
0.1
0.11
−15 dB
0.1
0.11
−25 dB
−0.05
0
−25 dB
0.05
−0.05
(a) DAS
0
0.05
(b) Capon (-/SB)
25 dB
0.04
0.05
25 dB
0.04
0.05
15 dB
0.06
15 dB
0.06
5 dB
0.07
0.08
5 dB
0.07
0.08
−5 dB
0.09
−5 dB
0.09
−15 dB
0.1
0.11
−15 dB
0.1
0.11
−25 dB
−0.05
0
0.05
−25 dB
−0.05
(c) IAA (MB/SB)
0
0.05
(d) IAA (MB/MB)
Figure 5. Resulting images after beamforming simulated speckle data of scenes containing hollow cyst-like structures. The subfigure captions indicate the
applied approach. In a) we have marked the region from where the image cuts of Figure 6 is taken, as well as the regions used in calculating the CNR in
(16). The IAA (SB/SB) image (not shown) has a strong visual resemblance with that of the IAA (MB/SB). Similarly, the IAA (SB/MB) and Capon (-/MB)
resemble that of the IAA (MB/MB).
Table I
S TATISTICS FROM 33 SIMULATED CYST IMAGES , CF. F IGURE 5. T HE CYST WIDTHS IN MM ARE FROM A CUT AT 8 CM RANGE , STRAIGHT THROUGH TWO
CYLINDRICAL CYSTS WITH RADII 10 MM AND 20 MM .
Approach
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (-/SB)
Capon (-/MB)
µ/σ
1.88 ±0.01
1.85 ±0.01
2.74 ±0.02
1.79 ±0.01
2.77 ±0.02
1.81 ±0.01
2.56 ±0.02
CNR
4.00 ±0.03
4.99 ±0.03
6.27 ±0.05
4.75 ±0.04
6.30 ±0.05
4.34 ±0.03
6.02 ±0.04
has been reduced in these images, the contrasts as measured
by the CNR in (16) have not.
The other columns of Table I show estimates in mm of
the width of the cysts at a range of 8 cm. The segmentation
was done by applying a threshold on the mean images that
is halfway between the minimum and maximum of the scanline values in dB for each approach. The standard-deviation
measures stem, as for the SNR and CNR, from statistical
bootstrapping. We see that the IAA methods all improve on
the DAS BF, as the estimated widths are closer to the correct
one and two cm, respectively. The Capon BF (-/SB) seems to
do barely better than the DAS BF on this criterion. However,
reducing the loading factor in the Capon BF would give wider
cysts. Note that the cyst widths are all underestimated because
there is always a certain width of the effective “point-spread
function”.
Cyst 1 width
7.6 ±0.07
8.1 ±0.09
8.5 ±0.09
8.6 ±0.03
8.1 ±0.08
7.6 ±0.04
8.7 ±0.08
Cyst 2 width
17.3 ±0.10
18.0 ±0.05
18.7 ±0.03
18.6 ±0.09
18.2 ±0.10
17.5 ±0.13
18.7 ±0.08
Cuts through the mean images at a range of 8 cm can be
found in Figure 6. The figure shows that the IAA gives a
more correct placement of the cyst border than the DAS BF.
The IAA (xx/SB) cuts also show a lower, and more correct,
amplitude level just inside the cyst. The Capon BF (-/SB),
however, improves only slightly on the DAS BF. Again, this
would improve with a lower diagonal loading factor.
C. Phantom data
Resulting images stemming from processing real, recorded
ultrasound data can be seen in Figure 7. Parts of azimuthal
scanlines at ranges 6 and 8.2 cm from such images can be
found in Figure 8. The first cut is through the central cyst,
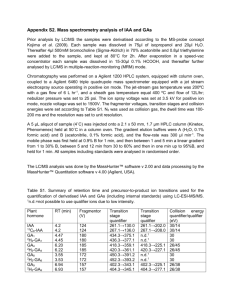
while the other is through two point reflectors. SNR and CNR
values are listed in Table II.
7
25 dB
0.04
25 dB
0.04
15 dB
0.06
15 dB
0.06
5 dB
0.08
5 dB
0.08
−5 dB
0.1
−5 dB
0.1
−15 dB
0.12
−15 dB
0.12
−25 dB
−0.06
−0.04
−0.02
0
0.02
0.04
−25 dB
0.06
−0.06
−0.04
−0.02
(a) DAS
0
0.02
0.04
0.06
(b) Capon (-/SB)
25 dB
0.04
25 dB
0.04
15 dB
0.06
15 dB
0.06
5 dB
0.08
5 dB
0.08
−5 dB
0.1
−5 dB
0.1
−15 dB
0.12
−15 dB
0.12
−25 dB
−0.06
−0.04
−0.02
0
0.02
0.04
0.06
(c) IAA (MB/SB)
−25 dB
−0.06
−0.04
−0.02
0
0.02
0.04
0.06
(d) IAA (MB/MB)
Figure 7. Images created using beamforming of real, recorded ultrasound data. The phantom contains both point targets and cyst-like structures. The numbers
on the axes are in meters. The subfigure captions indicate the applied approach. Plots of image cuts at the indicated locations can be found in Figure 8.
Table II
M EASUREMENTS FROM THE PHANTOM DATA .
Approach
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (-/SB)
Capon (-/MB)
SNR
1.88
1.89
2.41
1.83
2.37
1.89
2.39
CNR
3.93
4.29
4.00
4.27
4.12
4.11
4.25
All the adaptive approaches produced images with more
sharply indicated point targets compared to what is produced
by the non-adaptive DAS BF. The DAS BF and the three
(xx/SB) versions (only two shown) produce visually quite
similar results, although, as in the simulations, the point-targets
are smaller and more sharply defined in the Capon BF and
IAA outputs, with the Capon BF producing even sharper pointtargets than the IAA. The peak amplitude is, however, reduced
for the Capon BF; something that an increased diagonal
loading factor would alleviate, although at the cost of a slightly
narrower cyst and wider point target renderings. The three
(xx/MB) versions (only one shown) smooth the speckle pattern
while retaining sharp edges along the periphery of the cyst.
The disadvantage, however, is a slightly reduced contrast compared to the (xx/SB) versions. This is both visually apparent
and can be seen by comparing CNR values.
From the azimuthal image cuts (Figure 8) we see that
the DAS BF has produced amplitude peaks that look like
sidelobes just inside the central cyst. All (xx/SB) versions have
dampened those peaks substantially. As in the simulations, the
amplitude of the IAA inside the cyst is generally lower than
both the Capon BF and the DAS BF. We can also see that
the (xx/MB)’s give spatially smoother outputs, while it is still
making a rather sharp transition at the edges of the cyst.
In Figure 9 we see azimuthal image cuts stemming from
experiments where we have discarded every other recorded
beam before running the algorithms. The image cuts show
strong similarities to the corresponding all-beam data in Figure
8. However, it is apparent that the number of image samples is
reduced; e.g. the larger distance between the samples causes
the peak at about −10◦ to be a bit less sharp.
D. Convergence and computational considerations
The number of iterations before convergence when using
SB in the IAA was about twice that needed when applying
MB. The SB needed between ten and fifteen iterations while
the MB required only five to ten for the per-iteration modelupdates to be practically zero. The Capon BF (both SB and
MB) requires roughly the same amount of computations as a
single IAA iteration, and is hence much faster than any of the
IAA methods.
VI. D ISCUSSION AND CONCLUSION
From the results we can conclude that the IAA in combination with our proposed time-delay and phase-shifting of
the data has great potential in medical ultrasound imaging.
Compared to the conventional DAS BF, the approach produces
images that show a better point-target resolvability and display
more geometrically correct and more well-defined cyst-like
8
10
30
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
25
0
20
15
Amplitude (dB)
Amplitude (dB)
−10
−20
−30
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
−40
−50
−8
−6
−4
−2
0
2
4
6
8
10
5
0
−5
−10
−15
−20
−22
10
−20
−18
−16
−14
−12
−10
−8
Azimuthal angle (deg)
Azimuthal angle (deg)
Figure 8. Image cuts from the images in Figure 7. The cuts intersect a hollow cyst at 6 cm range (left pane) and two strong point scatterers at 8.2 cm range
(right pane).
10
30
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
25
0
20
15
Amplitude (dB)
Amplitude (dB)
−10
−20
−30
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
−40
−50
−8
−6
−4
−2
0
2
4
6
8
5
0
−5
−10
−15
10
Azimuthal angle (deg)
Figure 9.
10
−20
−22
−20
−18
−16
−14
−12
−10
−8
Azimuthal angle (deg)
Similar image cuts as in Figure 8, although from experiments where we have used only half the number of transmitted and received beams.
structures. The approach is intuitive, needs no tuning of user
parameters, and is easily implemented. The disadvantage,
however, is the increased computational load.
When comparing the IAA to the non-iterative Capon BF,
the IAA shows a similar two-point resolving power and also
seems to produce more sharply-defined and more geometrically correct cyst-like structures. Furthermore, the IAA is
less prone to amplitude loss caused by signal cancellation.
Signal cancellation is more pronounced in scenarios where
wider transmit beams are applied, e.g. to increase frame-rate,
as contiguous transmitted beams illuminate closely located
targets more similarly in this case. In addition, the IAA has
no free parameters, while the Capon BF relies on a diagonal
loading parameter to function properly. User-parameter free
versions have been developed, see e.g. [14], although they
have not yet been demonstrated to remedy the challenges
with signal cancellation in medical ultrasound imaging. The
advantage of the Capon BF over the IAA, however, is computational cost, as there is for the Capon BF no need to
iterate, causing it to be about five to fifteen times faster
in straight-forward implementations since each IAA iteration
is numerically close to performing the Capon BF. The use
of more efficient implementations, e.g. [19], could probably
reduce this gap considerably. Please see [11] for a run-time
example and a simple algorithmic complexity analysis of the
Capon BF.
Applying all the beams when estimating the model parameters in IAA, the (MB/xx) approaches, gives faster convergence,
although at the cost of slightly reduced point-target resolvability and some added geometric distortions when imaging
cysts. This might change, however, in scenarios where there
are fewer transmit beams and noisy measurements. The reason
why the (MB/xx) approaches require fewer iterations is not
fully understood, however it is probably linked to the reduced
9
1
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
0.9
Dip−to−peak ratio
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
0.5
1
1.5
2
2.5
3
Angle (deg) separating the two point targets
(a) Full Tx
1
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
0.9
Dip−to−peak ratio
0.8
0.7
0.6
0.5
0.4
0.3
noise, or reduced spatial variance, of the model coefficients.
In the experiments we used phased linear arrays. The IAA
method itself is however not limited to this array geometry,
and adaptations of the steering vectors in the model to handle
any geometry are trivial. The real-world performance of the
IAA in other array geometry settings has though not been
investigated.
Both the IAA and Capon BF lend themselves to form
compound images where the contributions from the different
beams are added together incoherently. We referred to this
as (xx/MB), and the results show that the approach reduces
speckle variance while it is still able to produce images that
have good point resolvability and displays cyst-borders rather
well. The negative aspect is that the overall dynamic range is
reduced. The contrast in the image, though, as measured by
CNR, is kept, or slightly increased, for the IAA compared to
the DAS BF in such settings.
To harvest the full potential of high-resolution adaptive
BFs one must use a dense set of spatial samples. This high
number of samples can be the result of using a high number of
transmitted beams or it can come from upsampling at reception
(multiple line acquisition). We have, however, demonstrated
that the suggested IAA approach can be directly applied with
beneficial results even in sampling scenarios found in more
typical DAS BF-based scanners.
0.2
ACKNOWLEDGEMENTS
0.1
0
0.5
1
1.5
2
2.5
3
Angle (deg) separating the two point targets
(b) Half Tx
Figure 4. Two-point resolving power. On the y-axis: The ratio between the
minimum amplitude value between the two point reflectors and that of the
peak value. a) and b) show imaging setups as in Figure 3
5
DAS
IAA (MB/SB)
IAA (MB/MB)
IAA (SB/SB)
IAA (SB/MB)
Capon (−/SB)
Capon (−/MB)
0
Amplitude (dB)
−5
−10
−15
−20
−25
−30
−35
−40
−45
11
12
13
14
15
16
17
18
19
Azimuthal angle (deg)
Figure 6. Azimuthal image cuts from the mean of 33 speckle realizations of
the scene behind the images in Figure 5. For the exact location, see Figure 5a).
The dashed vertical line indicates the border of the cyst.
We wish to thank Dr. Anders Sørnes, GE Vingmed Ultrasound, for providing the experimental ultrasound phantom
data.
R EFERENCES
[1] B. Steinberg, Principles of aperture and array system design: Including
random and adaptive arrays. New York, Wiley-Interscience, 1976. 374
p., 1976.
[2] B. Van Veen and K. Buckley, “Beamforming: A versatile approach to
spatial filtering,” ASSP Magazine, IEEE, vol. 5, no. 2, pp. 4–24, 1988.
[3] F. Vignon and M. Burcher, “Capon beamforming in medical ultrasound
imaging with focused beams,” Ultrasonics, Ferroelectrics and Frequency
Control, IEEE Transactions on, vol. 55, no. 3, pp. 619 –628, march 2008.
[4] J. Mann and W. Walker, “A constrained adaptive beamformer for
medical ultrasound: initial results,” in Ultrasonics Symposium, 2002.
Proceedings. 2002 IEEE, vol. 2, oct. 2002, pp. 1807 – 1810 vol.2.
[5] M. Sasso and C. Cohen-Bacrie, “Medical ultrasound imaging using the
fully adaptive beamformer,” in Acoustics, Speech and Signal Processing,
2005. Proceedings (ICASSP’05). IEEE International Conference on,
vol. 2, 2005, pp. 489–492.
[6] F. Viola and W. Walker, “Adaptive signal processing in medical ultrasound beamforming,” in Ultrasonics Symposium, 2005 IEEE, vol. 4.
IEEE, 2005, pp. 1980–1983.
[7] Z. Wang, J. Li, and R. Wu, “Time-delay- and time-reversal-based robust
capon beamformers for ultrasound imaging,” Medical Imaging, IEEE
Transactions on, vol. 24, no. 10, pp. 1308 –1322, oct. 2005.
[8] J.-F. Synnevag, A. Austeng, and S. Holm, “Adaptive beamforming
applied to medical ultrasound imaging,” Ultrasonics, Ferroelectrics and
Frequency Control, IEEE Transactions on, vol. 54, no. 8, pp. 1606 –
1613, august 2007.
[9] I. Holfort, F. Gran, and J. Jensen, “Minimum variance beamforming
for high frame-rate ultrasound imaging,” in Proc. IEEE Ultrason. Symp,
2007, pp. 1541–1544.
[10] J. Synnevag, A. Austeng, and S. Holm, “Benefits of minimumvariance beamforming in medical ultrasound imaging,” Ultrasonics,
Ferroelectrics and Frequency Control, IEEE Transactions on, vol. 56,
no. 9, pp. 1868–1879, 2009.
10
[11] A. Jensen and A. Austeng, “An approach to multibeam covariance
matrices for adaptive beamforming in ultrasonography,” Ultrasonics,
Ferroelectrics and Frequency Control, IEEE Transactions on, vol. 59,
no. 6, pp. 1139–1148, 2012.
[12] T. Yardibi, J. Li, and P. Stoica, “Nonparametric and sparse signal
representations in array processing via iterative adaptive approaches,”
in Signals, Systems and Computers, 2008 42nd Asilomar Conference
on. IEEE, 2008, pp. 278–282.
[13] T. Yardibi, J. Li, P. Stoica, M. Xue, and A. Baggeroer, “Source
localization and sensing: A nonparametric iterative adaptive approach
based on weighted least squares,” Aerospace and Electronic Systems,
IEEE Transactions on, vol. 46, no. 1, pp. 425–443, January 2010.
[14] L. Du, T. Yardibi, J. Li, and P. Stoica, “Review of user parameter-free
robust adaptive beamforming algorithms,” Digital Signal Processing,
vol. 19, no. 4, pp. 567–582, 2009.
[15] J. Jensen, “Field: A program for simulating ultrasound systems,” in 10th
Nordic-Baltic Conference on Biomedical Imaging, vol. 4, supplement 1,
PART 1: 351–353. Citeseer, 1996.
[16] J. Jensen and N. Svendsen, “Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers,” Ultrasonics,
Ferroelectrics and Frequency Control, IEEE Transactions on, vol. 39,
no. 2, pp. 262–267, 1992.
[17] H. Van Trees, Optimum array processing. Wiley Online Library, 2002.
[18] D. Shattuck, M. Weinshenker, S. Smith, and O. von Ramm, “Explososcan: A parallel processing technique for high speed ultrasound
imaging with linear phased arrays,” The Journal of the Acoustical Society
of America, vol. 75, p. 1273, 1984.
[19] G. Glentis and A. Jakobsson, “Time-recursive iaa spectral estimation,”
Signal Processing Letters, IEEE, vol. 18, no. 2, pp. 111–114, 2011.