PFC/JA-83-2 HIGH RESOLUTION X-RAY SPECTRA FROM MOLYBDENUM ... C Plasma Fusion Center
advertisement

PFC/JA-83-2
HIGH RESOLUTION X-RAY SPECTRA FROM MOLYBDENUM IONS
IN THE ALCATOR C TOKAMAK
E. Killne, J. Killne and Robert D. Cowan
Plasma Fusion Center
Massachusetts Institute of Technology
Cambridge, MA
02139
January 1983
This work was supported by the U.S. Department of Energy Contract
No. DE-AC02-78ET51013. Reproduction, translation, publication, use
and disposal, in whole or in part by or for the United States government is permitted.
By acceptance of this article, the publisher and/or recipient acknowledges the U.S. Government's right to retain a non-exclusive,
royalty-free license in and to any copyright covering this paper.
High Resolution X-ray Spectra from Molybdenum Ions
in the Alcator, C Tokamak
E. Kllne and .. Kllne
Harvard-Smithsonian Astrophysical Observatory
Cambridge, MA
02138
and
Robert D. Cowan
Los Alamos National Laboratory
Los Alamos, NM
We report on measurements of the X-ray
region
87545
line
emission
in
the
wavelength
X = 4.3 to 5.3 A from molybdenum in charge states between 28+ and 32+.
The experimental results
were
compared
with
calculated
line
spectra
(also
presented)
which facilitated the identification of many of the principal 2p-3d,
2s-3p
2p-3s
and
transitions.
contributions were also found.
the dominant ones.
charge
states
for
Some
indications
of
weaker
line
The principal 2p-3d transitions were found to be
In particular, Mo3 2 + gave stronger contributions than
our plasma condition of T e
= 2.5 to 3.0 keV.
other
1.0 to 1.5 keV while available
calculations of relative ion abundances have predicated Mo32+ to
strongest at T
satellite
be
relatively
Page 2
Introduction
impurity
Characteristic X-ray emission from highly ionized
plasmas
is
a
source
of
information
elements
for
instance,
can
reveal
the
relative
Z
elements
rates /1/.
while
importance of competing
transported
limiters
molybdenum,
of
the
High Z
fully
observe
for
vacuum
vessel,
impurities,
such
with Z = 42, will be only partly stripped of their electrons at
electron temperatures (T ) of a few keV, common in tokamaks,
more
to
into the plasma core, undergo successively increasing
ionization mainly through electron impact collisions.
as
in the
due to a strong Z dependence in the dielectronic recombination
Impurity ions from the walls and
being
hot
The emission from high
Ionization-recombination processes which otherwise is difficult
low
in
on the ionization stages present
plasma and is a means to diagnose the plasma conditions.
Z
atoms
stripped
low
Z
elements.
in contrast to
the
The high Z elements have a large total
excitation cross section and the subsequent radiative de-excitations
can
cause
large energy losses and eventually lead to major disruptions of the plasma.
stability and energy confinement of the plasma is therefore highly sensitive
The
to
the amount of high Z impurities and the atomic properties of the species present
/2/.
In order to estimate the overall energy losses due to impurity
modeling
of
the
plasma
radiative
available atomic cross sections /2,3/.
different
ionization
is not well known.
equilibrium
has
it
done
using the best
of
the
and excitation processes to the ionization stage build-up
Furthermore, the general assumption of a plasma
not
is
been
the relative importance of
However,
in
coronal
been verified by experiments for tokamak plasmas, nor has
the assumption that heavy impurities will
Therefore,
has
processes
radiation,
utmost
importance
be
to
in
ionization
identify
responsible for heavy radiation losses in the plasma.
the
equilibrium
emission
/4/.
spectra
Page 3
The spectra of heavy, partly stripped ions
molybdenum
at
temperatures
of
a
few
keV,
of
high
are
Z
ion
can
be
calculated
supporting experimental
spectroscopic
with
of
some confidence only if there is available
high
Z
relative complexity of the spectra requires,
energy
resolution
elements
first
in
plasmas
of all,
spectrum
in order to separate and identify individual emission lines.
consists
of
(bremsstrahlung)
simultaneous
since
background
emission
resolvable
calculated spectra.
concerning
allowing
For these
cases,
line
emission
identification
important
with
conclusions
on
spectra
the
be
help
the
ions
are
of
reached
the major ionization and excitation processes of these ions embedded
In this paper we
measurements of the X-ray line emission from molybdenum impurites in
the plasma of the Alcator C tokamak /10/ in the
which
can
on
for
However,
/9/.
in the hot plasma usually consisting of hydrogen or deuterium.
report
the
emission from several successive
characterized by closed shell atomic configurations, the
experimentally
The
experiments with high
ionization stages which produces quasi-continuous features superimposed
continuum
recent
/6,7,8/.
Even so, all cases are still not amenable to spectral investigations
measured
as
The line spectrum of
This has been demonstrated in some
information.
investigations
such
composed of lines from many
multi-charge states each having a complex level structure.
each
elements
covers
strong
n=2
to
wavelength
region
4.3-5.3 A,
n=3 transitions from molybdenum in neon-like and
adjacent charge states.
2.
The experiment was performed
Alcator
C
located at MIT /10/.
Experimental
at
the
high
field,
density
tokamak
The magnetically confined plasma is physically
limited by two molybdenum rings (radius 16 cm) inside
X-ray
high
the
vacuum
vessel.
An
spectrometer views the plasma along a central chord at one of the limiter
Page 4
ports with an instrumental vertical aperture
central
part of the plasma.
window
tokamak
and
of
the
cylindrically
to
-5 cm
60 cm
from
thickness 0.04 mm at the slit
spectrometer.
The
spectral
the
plasma
the
separates the vacuum ot the
analysis
cylinder
axis.
The
photons
is
done
electronics.
a
proportional
counter
PDP11/30
computer.
acquisition
system
shots.
and
The useful bandwidth or
the full wavelength region
X = 4.3-5.3
appropriately
the
positioning
at
the
In this way an extendea spectrum can be
recorded simultaneously for individual plasma discharges and partially
between
parallel
A multichannel analyser stores and displays tne spectrum
before the data are transferred to the common data
using
a
with
are counted with a position
sensitive detector consisting of a 10 cm single wire
tokamak
A
curved, (R = 58.5 cm) Bragg diffracting crystal (pentaerytnritol,
horizontal
standard
the
center.
PET, 2d0 0 2 = 8.742 A) which disperses the photons along the direction
to
of
The incident photon flux is defined by an entrance
slit (0.2 mm wide) which is located about
beryllium
corresponding
analyzed
-s.h such spectrum is about 0.4 1 and
1
of the spectrometer
detector.
was
covered
by
Other details about the experimental
set up have been discussed in earlier papers /11,12/.
The X-ray emission in the 4.3-5.3
conditions
however,
of
the
main
plasma
1
region can be
parameters.
The
studied
under
molybdenum
is measurable only under rather limited plasma conditions
varying
line emission,
because
molybdenum
we
deuterium.
The impurity concentration is therefore controllable but found
high
for
only
density N
C).
observe
low
is
an
ambient
the
impurity in the plasma of hydrogen or
to
be
densities and seems to rise sharply with decreasing electron
(or increasing Te, since Te and N
are inversely correlated in
Alcator
The impurity level of molybdenum ions seem to have a different plasma depend-
ence than has that of low Z impurities, such as sulphur (see Fig. 1).
Thus,
the
Mo line spectra measured here represent a rather narrow band of plasma parameters,
Page 5
namely electron densities of Ne
interferometer
1.5 to 3 x 1014 cm
(as determined from laser
a 1.0 keV to 1.5
measurements) and electron temperatures of T
keV (as determined from the X-ray continuum measurements).
The plasma
current,
electron
density,
over a period of' some
were
measurements
as
conditions
and
parameters
as
such
plasma
electron temperature are essentially constant
100-200 msec
electronically
constant part of the discharge.
by
characterized
in
the
middle
to
gated
pick
of
the
discharge.
Our
out the emission during the
Hence, the time scale of observation
was
long
compared to typical ionization-recombination times.
An example of the time history for a typical plasma discharge is
Figure
2.
Here
we
can
compare
the
time
shown
in
dependence of plasma current and
electron density with the signal from the integrated (hv >1 keV) X-ray
emission
Our signal for- this discharge represents largely the time
and our X-ray signal.
dependence of the Mo line emission over the wavelength region 4.5-4.9 X.
of
The time dependence of the X-ray emission over the wavelength band
detector
(A = 0.4 X) was recorded for each discharge, with a time resolution
of typically one msec.
the
molybdenum
current.
where
As exemplified in Figure 2, the temporal
emission
was
generally
found
ohmic
to
follow
the
of
However, at the time in the middle
comparison
of
the
so-called
for
used,
instabilities.
msec)
(T ~180
Mo
line
An interesting observation can
Another
sawtooth
instance,
temporal
to
detect
feature
of
the
the
made
we
in
total (broad-band) X-ray
the
and
emission
be
The total
characteristic
plasma
emission
m=1
discharge
instability with a time period of some 10 ms.
is
plasma
is
the
The presence
of this instability is generally detected in the broad band X-ray emission,
here
of
that of the plasma
discharge
emission, both measured along the central plasma chord.
commonly
dependence
heating is applied the Mo-emission shows a strong rise compared to
relative moderate rise in current.
the
the
and
demonstrate that this can be seen also in the molybdenum line emission
Page 6
(Figure 2).
to
the
Moreover, since the-sawtooth instabilities are believed
core
region
of the plasma -inside the m=1 surface,
line of sight (limited vertically to the central 5 cm
important
contributor
profiles.
While the sawtooth instabilities are seen
highly
ionized
to
the
molybdenum,
measured
they
are
of
broader
spatial
profiles
belong
this portion of our
the
plasma),
is
an
emission from the radial impurity ion
not
as
in
of
these
our
apparent
helium-lik'e ions (such as chlorine or sulphur), which
expectedly
to
is
ions.
emission from the hydrogen-like sulphur (having a higher
signal
from
the
in the emission from
consistent
with
the
Yet, the time resolved
ionization
potential)
again shows a time behavior with sawtooth instabilities; the X-ray emission from
S and C1 has been discussed earlier /11,13,14/.
3. Theoretical Calculations
Previous experimental work on the X-ray line emission in molybdenum has led
to
the
identification
of
Mo
XXXIII lines at 4.415, 4.630, 4.804, 4.982, and
5.207 A /15,16/.
These identifications are reasonably firm and can be used
support
interpretation
of
the
of
other
lines in Mo spectra with the aid of
theoretical calculations employing the Slater-Condon theory of atomic
/17/.
that
effects.
and
structure
One-electron radial wave functions were computed via the HFR method /18/,
which uses Hartree-Fock equations with inclusion
terms
in
of
mass-velocity
and
provide first order corrections for (spin-independent) relativistic
The Hamiltonian matrices were evaluated using these radial
diagonalized
to
give
eigenvalues
(energy
levels)
and
functions,
eigenvectors
(N-electron, intermediate-coupling wavefunctions) in the usual -way.
purpose,
all
Coulomb
radial
the
Eav)
a
scaled
down
For
this
integrals F , Gk, and Rk (contributing to matrix
elements through terms other than
were
Darwin
by
configuration
factor
of
center-of-gravity
0.93
to
allow
for
energy
weak
configuration-interaction effects and thereby improve agreement between computed
Page 7
and
observed wavelengths;
the Blume-Watson
spin-orbit radial integrals
C
were
similarly scaled by a factor 0.98.
Configuration
the
near
interactions of the type 3s3d+3p2 are
equality
energy
in
of
the
important
because
corresponding configurations,
of
and were
included where pertinent, as in calculation of the transition arrays
No XXXII 2p 63s - 2p 53s3d+2p 53p2
and
Mo XXXI 2p 6 3s
Trial
2
- 2p 5 3s
calculations
unimportant,
2
3d+2p 5 3s3p 2
showed
other
configuration
interactions
2p-3s,
and
2p-3d
Ne-like to Mg-like molybdenum are listed in Tables I and II.
given
in
configurations,
[,
the
coupling
representation
excitations
in
Level designations
two-open-subshell
for
and in the
2 3 11
for three-open-subsbell cases.
best
(especially
Ij2
the
(L2 3 S2 3 )
representation
are
be
and most of these were neglected.
The principal lines computed for 2s-3p,
are
to
possible
where
ones
3s3d+3p2
to
use,
Although
basis-state
configuration
mixings
these
representations
mixings are usually large
are
present),
and
level
designations must consequently not always be taken literally.
Calculations were also made
excited
states
satellite-line
in
Al-like
transition
for
and
arrays
analogous
Si-like
produced
transitions
molybdenum,
as
the
and
result
of
collisionally
for
the following
of
dielectronic
rec omb ina t ion:
No XXXII 2p 6 n1 - 2p5 n13d
Mo XXXI
2p6 3sn1
No XXX
2p 6 3s 2 n1
2p5 3sn13d
-
-
2p53 s2 n13d
(n1
3s,3p,3d,4s,4p,4d,4f)
Page 8
- 2p5 nl3s
No XXXII 2p 6n
(nl - 3s,3p).
The results are too complex and voluminous to present here,
of relatively little
and are in any
case
importance in interpretation of the strong observed lines.
The above results for Ne-like through Si-like molybdenum have been used
synthesize
spectra,
using
dielectronic-recombination
including
Doppler
and
III,
results.
IV,
methods
of
computing
excitation
and
rates to obtain intensities of the various lines,
and
instrumental
discussed elsewhere /19/.
Tables
siml1e
to
line
broadening.
Some details have been
The results of these calculations
are
presented
in
and V and in Figures 4-8 for comparison with the experimental
Computed wavelengths are accurate
to
about
+0.1
percent,
so
that
identification of observed lines can be considered fairly firm provided computed
and observed wavelengths agree to within +0.005 A.
While it
compare
in
computed
and
observed
intensities
order
is
necessary
to
identifications based on wavelength, quantitative agreement cannot
because
of
the
rough
collisional
excitation rates employed /20/,
to
corroborate
be
expected
neglect of
cascades and autoionization effects, possible non-equilibrium effects, etc.
4.
Results
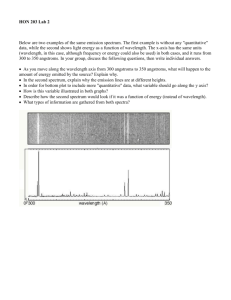
Figure 3 shows an example of the X-ray emission observed from the Alcator C
plasma
in the wavelength region 4.3-5.3 A.
which represents the
similar
plasma
full
shots
(normal)
were
used
In order to produce this spectrum,
bandwidth
of
the
spectrometer,
several
with three different detector settings.
The
plasma conditions were chosen so that the Mo emission was favoured although some
characteristic
emission
from
sulphur and chlorine impurities is also visible.
The spectra of helium-like chlorine and sulphur, and
along
with
/1.1!4/.
of
hydrogen-like
sulphur
their plasma diagnostics implications.have been discussed elsewhere
Page 9
Here we shall concentrate on the spectrum of
from
Figure
3
that
lying
in
i.e., 2s-3p, 2p-3d, and 2p-3s.
the
respectively,
is
obvious
Mo impurities in the plasma produce numerous lines in the
region 4.3-5.3 A, the identification of which we
transition,
It
molybdenum.
wavelength
regions
shall
by
type
of
These correspond to predicted lines
4.3-4.5 A,
4.6-4.9 A,
as summarized in Tables III, IV, and V.
are shown in Figures 4-8.
organize
4.9-5.3 A,
and
The measured line spectra
Each of these spectra covers part of
the. wavelength
region 4.3-5.3 A so that the 2s-3p lines appear in Figure 4, the 2p-3d lines in
Figures 4,
5 and 6, and the 2p-3s lines in Figures 6,
7 and 8.
Figure
8
shows
the results where the spectrometer was adjusted to reach 2p-3s lines beyond
X
=
0
5.3 A.
A.
Below we discuss the results in more detail.
The 2P-3d Transitions
Figure 5 shows the
4.6-4.9
1,
experimental
containing
2p-3d
and
calculated
transitions
in
,spectrum is from a single detector setting,
similar
plasma
discharges
to
spectra
molybdenum.
and
was
The
temperature
of Te
-1400 eV.
Mo
32+
an
/21/
and
Each distinct peak in the measured
assigned a letter and is similarly referred to in Table IV.
excitation
temperature T. = 1.2 ieV.
coronal
and
transition at 4.804 A /15/; wavelengths used for calibration
been
collisional
The plasma
cm- 3
X = 4.726 and 4.731 A
The theoretical spectrum shows the relative line intensities
the
twelve
0
2p-3d
has
over
To fix the wavelength scale we used the
are marked with an asterisk in Tables III-V.
spectrum
1014
region
experimental
provide better counting statistics.
H-like sulphur resonance transitions Is-2p at
the
the
accumulated
conditions were characterized by an electron density of 3 x
electron
in
equilibrium,
but
and
determined
by
dielectronic recombination rates for a plasma
It was assumed that the
the
as
relative
plasma
was
principally
in
population densities of the different
Page 10
vlarge states as predicted by the coronal approximation had
match
better
(see
intensities
experimental
our
to
be
The
below).
lines of the
calculated spectrum are identified by numbers which also are used in
Table
gives
IV
a
summary
wavelengths and relative
of
the
experimental
intensities and each
line
IV.
Table
theoretical results on
and
is
to
shifted
identified
to
as
ion
charge state and as to whether it is a resonance or satellite transition.
From the comparison
see
of experimental and theoretical results in Figure 5,
most of the observed prominent features can be understood in terms of
that
Of note is that the only strong lines that lack
2p-3d transitions.
counterparts,
i.e,
the
lines
is
within
theoretical accuracy of 0.1
about
5 mA,
percent.
The wavelength agreement for the
It
may
with the expected
consistent
i.e.,
be
that
noted
the
predicted
of the spin orbit components seems to be systematically slightly too
separation
large for each charge state, so
2p
integrals
that
the
theoretical
HFR values.
HFR
spin-orbit
radial
should have been scaled down by a factor 0.97 rather than 0.98.
For comparison, non-relativistic hF values of
than
theoretical
doublet at 4.73 A, have previously been identified as
the Is1 /2-2 p3 /2 1/2 doublet of H-like sulphur.
stronger
we
C2p
are
seven
percent
smaller
The experimental data clearly provide a sensitive test of the
theoretical assumptions.
At a more detailed level of the
line
identification,
we make the following observations.
Among the prominent lines of the Ne-like spectrum of Mo3 2 +
transitions
at
4.630 A
and
are
57gure 6),
states
in
line
2p-3d
4.804 A which are the predicted lines I and 16.
These lines always appear in plasma shots showing any Mo emission at
third
the
all.
The
the 2p-3d array, predicted to be at 4.855 A (line number 22 in
could contribute to the
observed
peak
(n)
although
other
charge
(Mo30+ and Mo 31+) might be even stronger contributors in this wavelength
region (cf.
Table IV).
The presence of Mo 3 2 + in the plasma, however, is in
no
Page 11
dowbt
and
it
is
further
corroborated
transitions as discussed below.
Ne-like
No
spectrum,
the
by
the
Besides these
theory
predicts
observation
three
lines
principal
contributions
satellites (lines No. 3,4,5,6, and 7 in Figure 5).
of strong 2p-3s
These
from
of
the
dielectronic
appear
on
the
long
0
wavelength
region
for
of the 4.630 A line and near the 4.804 A0 line.
side
In fact, the
X = 4.806-4.900 A contains a number of obse'rved peaks (i,j,k,l, and
instance)
which
each may contain one or several of these satellites.
potential satellite lines in this region are usually weaker than
short
wavelength
the calculations.
many
resonance
part
those
m,
The
in
the
of the spectrum, which is in qualitative agreement with
However,
besides these
dielectronic
satellites,
there
are
transitions of lower charge states of molybdenum to consider as
well.
The emission from Mo3 1+ produces a more complex spectrum since
transi.tion
seen
in
32+
Mo
can
be
split
into
several
for Mo3l+ two sets of peaks
and
together
according
higher atomic state.
Again,
to
Indeed,
4.804
X,
we
(b,d,e) and (j,k,l,m) that match up well to the
predicted lines (2,5,6) and (17,18,19,20) (Figure 5 and Table IV).
group
2p-3d
components.
corresponding to the two strong lines seen in M032+ at 4.630
see
each
the
the
2
p1/ 2 and
2
calculated
These
lines
p3 /2 spin-orbit separation of the
spin-orbit
separation
for
this
Na-like spectrum is systematically slightly greater than what is observed.
Finally, emission from Mo3 0 + is evidenced by the peaks at 4.680
at
X
(f)
and
4.854 1 (n) which we associate with predicted lines 7 and 21 of the Mg-like
spectrum (Fig. 5 and Table IV).
claimed
The latter identification, however,
be
with the same certainty due to the multitude of predicted lines and the
rather vague experimental peak profiles In this region.
notm
cannot
that
the
line f at 4.680
1
It
is
interesting
to
is always observed to be one of the dominant
featares in the spectrum of molybdenum emission,
along with the two strong No32+
Page 12
lines
and
4.630
at
4.804
A.
besides
Moreover,
the three main ionization
stages, Mo3 2 +, M031+, and Mo30+, we see contributions in the wavelength
X
4.685-4.800
and
4.880-4.950
I
could be attributed to emission from
which
lower ionization stages as indicated in Table
M029+
contributions
are
mostly
very
regions
IV.
However,
Mo2 8 +
these
and
weak compared to the generally dominant
M032+ lines.
We have thus been able to firmly identify the presence of , 32+, Mo3l+, and
The fact that Mo32 + dominates the emission for our plasma conditions is
go
surprising in view of current equilibrium calculations /4/ which
predict
that
No32+ should be the predominant charge state only at temperatures of T
= 2 to 3
keV, i.e., considerably higher than the diagnosed temperature of 1
1.5
for
Alcator
the
C plasma.
rates used in the calculated
ionization
of
states
states,
the
equilibrium
the
work we have corrected
tabulated
abundance
abundance
ions
In
conditions.
for
ratios
the
this
and
are
ratios
rates
are
are
too
for
used
relative
the
higher
too high or that the charge
distributed
not
low
to
according
coronal
atomic calculations presented in the present
apparent
discrepancy
adopted
semi-empirical
between
observed
abundance
charge
predict the relative intensities of the emission from different
we
keV
Our results therefore suggest that the ionization
recombination
molybdenum
to
and
ratios to
states:
abundances of .08, .13, .20, .25, and .17 for Mo 28+ through
32+ whereas the coronal equilibrium model at a temperature of 1.2 keV gives .18,
.27,
.13,
.07,
and .005, respectively /4/.
The relative intensities given in
Table IV have been normalized arbitrarily to the line at 4.804
X.
Page 13
L
2
The
P- 3 9 Transitions
region
Figure 7 shows the X-ray emission in the wavelength
where
4.9-5.2 A
of
spectrum
He-like
sulphur,
This part of
us with the wavelength calibration /21,22/.
provided
here
n=1
the Mo line emission spectrum is always dominated by the peak at 4.982 A.
peak
is
identified
with the calculated line 1 (i.e., a
Mo3 2+) as shown in Table V.
and
5.163
1
2p3/2-3s1/2
2
This
p1 / 2 -3s transition in
Except for the two peaks clearly observed at
5.155
most of the weaker features can also be correlated with calculated
2p-3s transitions.
The peaks at 5.207 and 5.217
transitions
in
1
can
be
accounted
for
as
32+
Mo
, the latter being of the magnetic quadrupole
These two lines always appear together in our spectra,
type.
This
we expect major contributions from 2p-3s transitions of molybdenum.
region also contains the well known n=2 to
which
of
but
are
observed
with varying relative intensities from one discharge to another.
32+
Having observed Mo
Mo3 1 +
lines, one
would
expect
and Mo3 o+ just as for the 2p-3d transitions.
manifestations
also
X
Peaks at 5.063 and 5.065
,may contain some contributions from predicted 2p1 /2 -3s transitions in
Mo3 0 +
in
wavelength region, whereas the corresponding transition in Mo3 1+ predicted
this
at 5.024
1
is very weak in our spectra and generally not detectable.
A spectrum bridging the wavelength regions of 2p-3s and
is
from
shown in Figure 6.
identified
as
discussed
before
relative intensities of 2p-3s and 2p-3d
broad
features
appear
ascribed to transitions
Table IV).
transitions
The keys in the figure refer to Table IV and Table V for
the (2p-3d) and (2p-3s) transitions, respectively.
been
2p-3d
in
from
the
the
region
lower
All
the
major
peaks
have
and this spectrum essentially shows the
transitions.
of
Other
X z 4.93-4.94 A
ionization
stages
rather
weak
and
which could be
Mo28+_
30+
(see
Page 14
in
A special effort was made to search for the predicted 2p-3s transitions
30+
Mo
No
and
31+
in
the
a
there
this
wavelength
region
fo3 1 +
Another peak appears at 5.274 A and beyond
this
is a broad structure extending to the -experimental cut-off at about
6
A 2p
5.31 A.
0
> 5.22 A, which is at the end of the
peak at 5.259 A which is very suggestive of the predicted
clear
2p3 /2-2 s1/2 line at 5.256 A.
peak
k
of
Figure 8 shows the result for
spectrometer bandwidth.
with
region
questionable
-3s
line in Mo
30+
is predicted at 5.304 A but it is
highly
Thus, this broad feature and the
that this weak line is observed.
5.274 A peak in the measured spectrum have not been theoretically accounted for
which
is
true
(Fig. 7).
32+
No
,
also
for the peaks (c, e, and f) at 5.049, 5.155, and 5.163 A
Of the predicted principal 2p /2 -3s1/ 2 and 2p3 / -3s1 /2 transitions in
31+
Mo
M
,and
30+
we
have
counterparts to the transitions
C.
2
not
been
able
p1 /2 -3s 1/2 in Mo
31+
to
localize experimental
30+
and 2p3 /2 -3 sl/2 in Mo
The 2s-3p Transitions
The observed spectrum in
Figure
4
distinct groups of line contributions.
already
been
4.40-4.55 A
/11,22/.
identified
contains
as
the
in
transitions
molybdenum.
in
The
this
The
region
peak
labelled
are
attributed
to
2s-3p
a can be identified with the
4.412 A,
while
the
2s1/2-3p1/2
seems to have no obvious experimental counterpart or is hidden under
the ls-2p lines from Cl 15+ .
.ransitomws
in
Mo
Peaks b
and
and
c
can
the
presence
of
be
accounted
for
by
2s-3p
there may also be some weak traces in the observed
spectrum of Figure 4 of the predicted lines 3 and
indicate
two
X >4.60 A have
molybdenum.
region
predicted 2s1/2-3p3 /2 transition in Mo 3 2 + at
transition
in
shows
lines of the He-like spectrum of Cl1 5 +
known
The remaining peaks seen
4.3-4.7 1
The lines in the region
2p-3d .transitions
well
X =
covering
M
.
However,
the
5,
where
Mo
the
and Mo
latter
would
lines have
Page 15
observed intensities relative to Mo
also
note
32+
that are much weaker than predicted.
We
that all 2s-3p transitions are observed to be quite weak relative to
the 2p-3d transitions which always dominate our emission spectra for molybdenum.
5.
We have shown that
molybdenum
of
calculated
charge
states
emission spectrum in the
observed,
sometimes
emission from other,
is
that
the
Discussion
2s-3p,
28+
appearing
and
as
Some of
quasi-continuum
the
the
dominant
principal
transitions
many
regions,
lower ionization states of molybdenum.
in
small
could
peaks
indicate
Another possibility
ionization states are 28+ to 32+ but with contributions
from dielectronic recombination excitation besides the
determing
2p-3d
to 32+ can account for most of the observed
4.3-5.3 A.
X -range
2p-3s,
transitions.
collisional
excitations
We have thus calculated the 2p-3d line
spectra for Mo2 8+ to Mo3 2 + to obtain an estimate for the effect of contributions
of doubly excited states reached through dielectronic recombination which can be
considered as the satellite line spectrum.
contributions from dielectronic
Table
VI
gives
a
VI)
are
first
compared
with
the
upper
spectrum
states
In
contribution
to
the
These
results
due to collisional excitation only and hence
(Column
especially for n=3 spectators.
A).
We
thus
note
that
spectrum
from
dielectronic
Although the
main
spectral
there
is
a
recombination,
features
observed
to emanate from radiative transitions from the collisonally excited states
there are other complex spectral features, particularly on the
side
Mo3 +.
case the spectator is limited to occupy n=3 states (column B in Table
singly excited
seem
and
and in the other it can occupy either n=3 or n=4 (column C).
significant
of
recombination to the 2p-3d transitions, where we
consider two cases of occupancy for electrons in Mo2 9 +, Mo 3 0 +
the
comparison
of the No
line at 4.804
X.
long
wavelength
The relative intensities of these spectral
Page 16
features are found to vary
between
different
discharges,
while
of the identified resonance transitions remain largely unchanged relative
those
to each other.
from
considerably
Vhiee
An example of this can be found in Fig.
consecutive single discharges.
9
which
shows
spectra
These intensity variations could be
attributed to temperature changes which would
show
up
most
strongly
in
the
relative intensities of the satellite lines.
In this context it is also interesting to consider the relative
of
importance
the dielectronic recombination radiation as part of the total X-ray emission
from the
molybdenum
calculated
ions
in
this
4.3-5.3
X
study,
cm- 3 with Nion
molybdenum
range.
As
an
illustration,
An=1 transitions in the wavelength region
102 cm
charge
3
states
.20,
.25,
The
ionization
(Mo2 8 +,
Mo2 9 +,
Mo3 0 +,
between
contributions
from
dielectronic recomb-ination.
the
=
0.8,
states
It is found
recombination is appreciable for the
for
distribution
abundance
Io31+,
the
o32+) we take to be
were
.08,
The results are shown in Table VII for
and .17, respectively.
three different temperatures (T
of
assumed the electron density to be Ne = 1014
We
independent of temperature and as given earlier, i.e., the fractions
.13,
we
the radiative power from dielectronic recombination relative to that
due to collisional excitation for
our
energy
1.2,
due
that
and
2.0
keV),
to
collisional
the
influence
distinguishing
excitation
of
and
dielectronic
An=1 transitions at these temperatures and
must be considered in determining the energy balance of the plasma.
With regard to the information on the X-ray
from
experiments
previous
we
note
line
spectrum
the
ours
in
fifteen
counterparts
molybdenum
the studies of laser induced plasmas /8/.
These spectra, which also cover the wavelength range 4.4 to 5.3
to
of
X,
are
similar
that the same transitions are found to dominate in both cases.
Mo
lines
found
in
reported
our
from
the
laser
work
there
spectra to all but two of the lines.
are
Of
matching
The lines at
Page 17
4.461 and 4.689
I
corresponding to Mo3 2 + 2s-3p and Mo3 1 + 2p-3d transitions
the latter can not be ruled
in our spectra or too weak to be detected;
missing
out since the relative line intensities are not always the same
the
laser
and
tokamak plasmas.
not previously reported
experiemnts
/16/.
in
are
in
spectra
of
On the other hand our spectra show many lines
either
the
laser
studies
or
in
exploding-wire
Of these lines we mention the magnetic quadrupole transition
at 5.217 X for which a relationship to plasma
conditions
can
be
identified.
The fact that this line is missing in other than the low density tokamak plasmas
can be seen as a manifestation of collisional depletion of the metastable
upper
state
laser
for
plasmas
of
higher
densities
such
as
those
produced
by
irradiation.
6. Conclusions
We have
measured
wavelength range
the help of
strongest
characteristic
X = 4.4-5.3
computed
I
are
is
theory
typically
5mx
spectra
which
observations
could
be
or
better
further
relativistic Dirac-Fock calculations
natural
part
of
the
theory.
are
molybdenum
also
The wavelength agreement
while
splittings tend to be systematically too large
latter
of
presented.
.ran be
calculating
mechanisms
referred
line
to
the
intensities.
the
The
the
relative
investigated
where
spin-orbit
calculated
to
observation.
The
by comparison with fully
spin-orbit
the
experiment
between
interaction
is
a
With regard to relative line intensities we do
observe quite large differences between measured and computed spectra
part
in
to be 2p-3d in Mo3 2 +, Mo3 1 +, and Mo3 0 + along
found
with 2p-3s and 2s-3p transitions.
and
emission
and interpreted the observed line spectrum with
Hartree-Fock
transitions
X-ray
rather
It
crude
still
excitation
seems
that
rates
additional
which
in
employed for
population
might be needed since differences between experiment and theory tend
Page 18
to occur also for the principal transitions of same ion charge states for
one
would
expect
the
line
intensity
to
be
determined by the ground state
excitation rate and hence simply by the oscillator strengths.
also
suggests
This
experiment
that the ratio of ionization to dielectronic recombination rates
for neon-like molybdenum and possibly other iso-electronic ions are larger
expected
based
which
on
the
than
observation of Mo3 2+ at temperatures of 1.0 to 1.5 keV
compared to the predicted relative peak abundance
of
Mo32+
at
a
factor
two
higher temperatures.
Acknowledgments
The collaboration with the Alcator group is gratefully acknowledged.
work was supported by the United States Department of Energy.
This
Page 19
References
1. A.H. Gabriel, Mon.Not.R.Astr.Soc. 160, 99 (1972).
2. D.E,
Post, et al, Atomic Data and Nuclear Data Tables 20, 397 (1977).
3. A.L. Merts, R.D. Cowan and N.H. Magee, Jr., Los Alam s National
Laboratory
Report LA-6220-MS, March 1976.
4. C. Breton,
C. DeMichelis,
M. Finkenthal,
and
M. Mattioli,
Association
Euratom CEA, ERU-CEA-FC-948, March 1978, C. Breton et al., EUR-CEA-FC-1159,
Sept. 1982,
J. Phys. B
(1983),
C. Breton,
C. deflichelis
and
and
M.
Phys.Rev. Lett. 41,
110,
1978,
J. Quant. Spectr. Radiat.
Mattioli,
Transfer 19, 367 (1978).
6. P.G. Burkhalter, J. Reader, and R.D. Cowan, J.Opt.Soc.Am., 67, 1521 (1977),
ibid 1-0, 912 (1980).
7. U. Feldman, Physica Scripta 24, 680 (1981).
N.J. Peacock,
8. H. Gordon, M.G. Hobby,
and
R.D. Cowan,
12,
J.Phys.B
881
(1979).
9. M. Klapisch in Production and Physics of Highly
Charged
Ions,
Stockholm
1982, to appear Physica Scripta T3, 1983.
10.
B. Blackwell et
Research Proc.
al
in
Plasma
Physics
and
Controlled
Nuclear
Fusion
9th, Int. Conf., Baltimore, 1982.
11.
E. K;llne, J. Kllne, and J.E. Rice, Phys. Rev. Lett. 49, 330, 1982.
12.
E. Kllne and J. K;llne, to appear, Physica Scripta T3, 1983.
13.
E. Kllne, J. Kllne, and A.J. Pradhan, to be appear Phys.Rev. A, 1983.
14.
E.
Kl11ne
Nio, 1693,
15.
and
AIP
J.
K;1lne,
Proceedings
Center
Series,
for
Astrophysics
Series
X-Ray and Atomic Inner Shell, Physics
E.V. Aglitskii, et al., Quantum Electron 1, 2067, 1974 and
Electr. A, 500 (1974).
Preprint
Sov. J. Quant.
Page 20
16.
P.G. Burkhalter, R. Schneider, C.M. Dozier, and R.D. Cowan, Phys.Rev., A18,
718 (1978).
17.
R.D.
Cowan, The Theory of Atomic Structure
and
Spectra
(U.
of California
Press, Berkeley), 1981.
18.
R.D. Cowan, and D.C. Griffin, J.Opt.Soc.Am. 66, 1010, 1976 and
R.D. Cowan,
.Opt.Soc.Am. 58, 808, 1968.
19.
R.D. Cowan, to be published in Physica Scripta T3, 1983.
20.
H.
21.
L.A. Vainshtein and U.I. Safronova, Atomic Data and
Van Regemorter, Astroph.
3.
136,
906 (1962).
21, 49 (1978).
22.
U.I. Safronova, Physica Scripta 23, 241 (1981).
Nuclear
Data
Tables,
Table I
-
A ()
Mo
Calculated Wavelengths and gf Values for Mo
2p-3s Transitions
pf
and
Classification
Classif ication
2s-3p
4.412
0.46
Mo 32+
4.458
0.17
Mo 32
4.425
0.18
Mo 31+
4.450
0.68
-
(2s1/2 , 3s3p 32
4.479
0.21
-
(2s1 /2 ,
4.500
0.12
-
(2s1/ 2, 3s3p
4.477
4.523
0.44
2s22p6 1 0
- (2s1 /2 2p 6 3p2
+
-
2s22p63s 2 1/2
(2s1 /2 2p6 3p1/2
1
3
- (2s1 / ,
2
"
0.16
1
- (2s1/2, 3s3p 1P
1 )1 /2
2s2 2p 6 3s2 1s
Mo 30+
4.482
Mo
2s-3p
s3p 3 1 1/2
P1 )3 /2
3 p / )1
3 2
- (2s1 / , 3p 1/2)
1
2
"
-
(2s1/ 2 ,
3p 3/2)2
-
(2p 51/2
3s1/2)1
-
(2p 53/2
31/2)1
-
(2p53/2
(M2)
2p-3s
4.980
5.204
0.09
mo32+
2p6 1 0
"t
0.12
5.212
to
5.024
0.048
5.256
0.091
5.064
0.060
Mo 31+
- (2p 53/2 *2
3s 1/2
63s 2 1/2
2p6 3s3p
3p2
5.067
0.057
5.069
0.024
5.079
0.042
5.111
0.054
0.16
3
0
p
i
It
0.044
0.103
3s 2 )3/2
- (2p 5 11/2
3s2 3p
3P
0
"o
3p 1
2
/ .
(2p 511 /2
3s2 31)3/2)
1
- (2p 5 11/2
3s 2 3p 1/2)
1
-
5.305
5.311
(2p 53/2
-
Mo30+
(M2)
3s1/2)2
-
(2p5 11/2 3s2 3p1/2)l
-
(2p 5 11/2
3s2 3p3/2)2
-
(2p 5 3 /2
3s2
-
(2p5 3 / 2 3s2 3p1/2 1
-(2p5 3/2
3
p3 / 2 ) 3
3s2 3p1/2)2
Table II
Mo
- Calculated Wavelengths and gf -Values for Mo
gf
4.627
1.71
4.802
1.84
"
- (2p53 /2 3d
5 /2 )1
4.855
0.001
i
- (2p53/2 3d
3/ 2 ) 1
4.628
0.74
4.659
1.15
4..665
1.36
4..693
0.09
4.813
0.78
Classification
Mo32+ 2p6 1 S0 - (2p5 1/2
3d3/2 )1
Mo31+
63s 2 1/2 - 0.65(2p 5 1/2 3s 3d 1D2 )3/2 +0.31(2p 51/2 3p2 1D2 3/
I
- 0.98(2p 51/2 3s3d 3 D1)1/2
of
I
it
"
0.81
of
"
4.834
Transitions
2p-3d
x
4.819
2p-3d
0.46
- 0.86(2p 5 /2 3s3d
11
D2 3/2
- 0.28(2p 5 /2 3s3d 3Dl$, 2) 3/2+0.69(2p 51/2, 3p
2 1D2V
11
1 3/2
1
3
5
2
1
3
- 0.48(2p 5 /2 3s3d D2 D3 ) 3/2+0.49(2p 3/ ,3p
D2 , P) 3/2
2
3
- 0.74(2p 5
3s3d 1D2 '3D2 )1/2 +0.20(2p 53/ ,3p2 1D
2
3 /2
2 )1/2
- 0.20(2p53/2 3s3d
"
D3 ,1D2 )3/2 +0.76(2p 5 / ,3 p2
3 2
1
3 2 3/ 2
0
4.842
1.18
- 0.52(2p 5 /2 3s3d 3D3 ) 3/2+0.44(2p 53/
3
4.860
0.36
- 0.66(2p 5 /2 3s 3d 3D2 '3D1 )1 /2 +0.29(2p 53/ ,3p2 1D '
1
2
3
2
4.674
1.30
4.688
0.05
4.696
0.06
Mo30+ 2p63s2
0.13
of
0.74(2p5/2
1s,2
2
D3
2s 3 P0 ) 3 /
2
2
1 /2
)
3s3p2 2
)1
2
2
2
2
0.91( 2 p 1/2' 3s3p 2 1 2 P /2,
P3 / 2 ) 1
/ '
1
5
it
it
0.80(2p 51/2' 3s3p2
"
4.752
,3p2
1 0 - 0.77(2p 5 1 /2 3s2 3d3/2 )1 +0.16(2p 5 1 /2 , 3s3p2
",
4.726
2
0.13
1/2,2D3 /2'2 1/2)1
2
0.79(2p 51/2' 3s3p 2D
"
2
,
4.850
0.95
4.866
0.74
0.40(2p 5 3 /2 3s2 3d5 /2 )1 +0.45(2p 5 / , 3s3p2
2P3 /2 )l
3 2
4.939
0.09
1 /2)1
0.50(2p 5
3 /2
2
3s 3d5 / 2 ,3/2 )1 +0.27(2p 5 3 / ,3s3p2 2p
2
3/2 )1
0.79(2p 53/2' 3s3p
P5 /2,2D5 /2 )1
Table III - A comparison of Experimental Wavelengths and Intensities in the X region
o29+
4.4-4.6 A with predicted Mo 2s-3p transitions from Mo
Mo
-
32+
Distinction is made between states excited by electron collisions and
dielectronic recombination.
Ionization Stages
Figure
Keys
x
exp
(A)
rel
exp
Figure
Keys
rel
,,
th
,
th ,
4.418
100
4.412
100
32+
4.424
47
4.426
28
31+
C115+ Us2
4.444*
4.450
4.458
110
37
4.468
135
30+
C1
4.497*
53
4.500
20
31+
4.510
35
29+
4.523
41
30+
*Calibration lines from Refs. 21, 22.
S0 -ls2p P1 )
32+
C115+
4.478
diel. recomb.
31+
4.464.:
4.503
diel
Coll)
Res. trans.
2 1S
0 -is2p P2
I
(1s2 1S0 -is2s 3
I
1)
.%
-4.
0.
(,44
0
0
'-4cy
CV C).
0l
0
0
+nCl
m cn
)
0>
M4
M(n
(Y)
M.
,c
C4%0 %0I 1
0
4lJ
0)0
C;)
000
00
ce)
(
C--iCN
PL4
P
-1 -
p4 C4
0.40.1
N
00
CYcv
cn'
4.-
-4
.0 coco
C6 04
-- 4
L0
f
990
740
H-i
W E
CN4CN
0
0
4
(n
'-4
+e+m
--
C;N
7
CY)
'-4
4-'4-
10
0
'
M4
M
r4
a)
al)
-1
11%.
-P
0
w5 m
+ 4
+I
.4<
*
++
_I
4-J
IN
00o~-~a.o~
++
,A4-4
M C(P) Vl) N Cv'
Lr LA
C1
$4
m
a)<t
4-
a) c
00r-
ON
4 -4-4
~
~
0
m
v
r- r-4
0-L
m
0
c'
m~
NY n
CYN
1
N
mC1
N
C,
N
a 0
'-40D
,
.o
l) -T
C')
. 0
.c4
C14
m' mO -',ci.c o~~ 'r-40
-1 .4
o m w
00
i-s
4
co)
Ln
%0
co
00
-4
+
0
.
0cl
0ML
~
-tmmO
a
Lnc 00
1
MIDM0MN0L
-mL
-
0
Q)
E
p
x
0
LA
-4
a)
1
1 V
tL)%0
r
0(O
AC4C)t
-
r
cc
Li-c D
c
c
r-4o ,4 cMrt
o%0.--oco
mC
1;
0
5
0.
0
C4
rX4W
44
a)r-OC
p10)
0
~
0 r
m cn
C
0
,-4
C) C4 '0 -CV
1
- -
10
N 0
~l-tC)
It~ P~c%~4 c--4LA r-.c0
C414
r4
co
x0
0,40
ID-i1
-4-
-.
%C %-400
00) -" 0-0 coci
CCP1
00
%0
O
-cv-.
oON4N
0en4
- I-I4.tr
14-
rCD0
W~.-
0
0
o
k'T-700 ,
A
0
f-A
CD-4-
0
0)0
*r4
.~
~L4
-('a
c .0
u
0
0)
'44
.J'
-4
-r-) -L
A
a
.4
4J
N
04
cn
Ln
-%
I
04
-1-
Co
o
-H
0
-
t
Ln
CIS
H1
H
Co
H
Co
-.
eq
H
I4
-H
4-i
+
-r
4o
0W4
10
H
Cn
Coq
H/
C)
r-4
V3
Nn
N
Cl
H
p
C
-0'
to
+
H H( 0Y
0
Ir
t
N
C14
0
NY C1
14
M Cl
C14
^-
e
Cl
cn
0
cq
I
0-
cyl
+ 0
N
0<
N
Cl cO00
r0
0
ONO 000
o
0
C
o
00
TD
D
C
%
0
-.
O
N
U
00
H
N
"0
04
i
N
li
o
N
0
M
4.4
0
44i
-1
0
0
-4
4~4
0
(0
P
$4 t
4m
CoOe
Hl
1. Cl -r-
AI -
4
00 ON
C
o4
0
-1
E44
0
-H
O
4)
m
FC
"o
-<
-
p~~
~ 0O r-HI-LA
0)
0T
-T
C40Z.- n
00O
MC
0<tN 4Ao
M
-CK
co
w
co
E-4
04
0
o
~0
X
NCo
4
-:-
o0
Cl
N
D
_.
000
ALA
L
LA
Cl L
o 0 I0
V-4
00
LA
L
.- 4
LA
L
Ln
H-
f
rl-
\O
H-
0
N
P- r
aO
HI
Cl
LA
1 N 1 N N
LA
LA
LA
LA'
O
FN
LA
0
$4 CO)
ca
.0
C.
0
-44
CO
4
'H
-,
.!
-
4
CO
N1
LA
u
Table VT
-
A cG.mparison of contributions (T = 1.2 keV) for dielectronic recombination
satellites to the total line strength for Mo 2p-3d transitions for three cases:
Mo 32+, B) including Mo29+ - Mo31+
31+
29+
- Mo
3 spectator electrons; C) including Mo
A) collisional excitation only, Mo28+
satellites belonging to n
satellites belonging to n
Figure
=
-
3 and n = 4 spectator electrons.
ieys
(Figure 6)
Ath (A)
1
4.625
2
4.628
4- 633
3
A
22
6.7
-
B
25
C
25
6.7
-
6.7
26
4.643
1.8
2.4
4
4.648
1.8
2.2
5
4.659
10.4
17
19
6
4.665
12.2
13
14
7
4.674 - 4.675
18.7
24
25
8
4.689
9
4.693J
10
1.2
6.8
9.3
4.699
3.3
5.5
6.7
11
4.705}
3.5
5.0
6.5
12
4.
7 0 8J
4.715
5.4
13
4.723
1.0
1.0
14
4.726
4.3
4.3
4.3
15
4.740 - 4.751
2.5
3
5
16
4.802
24
24
17
4.813
7.1
7.1
18
4.820
7.2
7.2
19
4.833
4.2
11
13
20
4.842
10.7
16
22
21
4.840
14
20
20
22
4.855
23
4.858
3.4
7
7
24
4.866
10.8
20
22
24
4.880
26
4.887
7.2
6.1
4.874
25
11
-
4.883
2.4
3.8
4.2
2.8
2.8
4.5
8.5
4.893
.17
4.899
3.1
4.2
5.4
28
4.905
1.6
2.5
7
4.910
5.3
29
4.931
2.3
2.3
2.3
30
4.940
2.1
2.1
2.1
Table VII - Comparison of the calculated radiative power from states
excited by
electron collisions and dielectronic recombination for An=1
transitions -in the wavelength region
A = 4.3-5.3 A in plasmas of
Te = 0.8, 1.2, and 2.0 keV.
Mo Charge
State
2p-3d Transitions
28
Spectator
nI
+
0.0185
29+
29
29+
+0.0301
29+ +
30
Te = 0.8 keV
(coll)
(diel.rec)
3p,3d
4s,4p,4d,4f
+0.0459
31+
31
0.0452
0.0876
0.100
0.083
0.054
0.031
0.035
0.030
0.0737
3s,3p,3d
30 +4s,4p,4d,4f
30~
Radiated Power (W/cm )
Te = 1.2 keV
Te
2.0 keV
(coll)
(diel.rec) (coll) (diel.rec)
0.144
0.183
0.149
0.096
0.045
0.050
0.041
0.113
3s,3p,3d
4s,4p,4d,4f
0.120
0.032
0.222
0.096
0.035
0.060
0.029
31
0.0572
0.142
0.278
32+
0.0420
0.102
0.196
32 )
0.194
(28+ - 32 )
2
p-3s
0.007
0.016
0.029
(2+
0.026
0.069
0.140
(28
-
0.511
0.476
0.448
0.928
2p-3d
-
2s-3p
32+)
0.310
Page 28
Figure. Capt ions
Fig.
-nission intensity variations from S14+ (s-2p)
1
rdifferent electran densities,
Fig.
bottom
show:
(I )
current
resolution
X-ray.
expansions of
The traces from
Plasma density N,:
125 kA/div,
the
Soft
The
and
0.58 x 10
X-ray
14
emission
two
lowest
third
for
N
2 Time evolution of a typical plasma shot.
th'e
(2 p- 3 s)
and Mo32+
traces
fourth
cm
traces
to
top
-3
/fringe,
(h
are
the
>1
Plasma
keV),
25-fold
show
the
to
High
temporal
sawtooth
instability.
Fig. 3 A spectrum
different
Fig.
over
the
detector
full
wavelength
settings.
range.
Wavelengths
Composed
and
indicated directly at the strong
peaks.
spectrum were Ne = 2.5 x 101 4 cm~
and Te = 1.3 keV.
Plasma
from
three
identifications
are
conditions
the
for
4 A comparison of experimental and theoretical spectrum in the region
Mo
2s-3p and 2p-3d.
The observed peaks are keyed with letters
predicted ones with numbers referred to in Tables III
(2p-3d).
The
experimental
plasmas conditioLs of N
insert
spectrum
ee = 1.7 x
10
14
is
cm
-3
an
and
T
and the
(2s-3p)
addition
of
and
IV
of 2 shots at
- 1.4
keV.
The
shows the spectrum from a single plasma shot with only emission
from chlorine present.
Fig. 5 A comparison of experimental
region
of
Mo
and
2p-3d emission.
have been ke-yed with letters and
2eferred
to
im
Table IV.
mwrmalized to the peak
X-ray
spectra
in
the
The peaks in the experimental spectrum
the
calculated
peaks
with
numbers
The experimental wavelength scale has been
(h) at 4.804 A.
ad.Aition of 12 shots at N
Fig.
theoretical
=
The experimental
3.0 x 10 1 cm~
and T e
spectrum is an
1.4 keV
6 A comparison of experimental and theoretical spectrum in the region
of
Page 29
Mo(2p-3d)
(Table
IV)
and
(2p-3s)
(Table
V)
experimental spectrum is an addition of 20 shots at N
and T e
transitions.
= 1.7 x 10 1 cm
3
1.6 keV.
Fig. 7 A comparison of experimental and theoretical spectra in the
No
The
2p-3s transitions.
region
of
The observed peaks have been keyed with letters
and the theoretical predicted ones with numbers referred to in Table V.
The
experimental
10 1 4 cm
Fig.
3
is
an
addition
of 5 shots at N
= 2.3 x
m 1.4 keV.
and T
8 A comparison of experimental and theoretical spectrum in the region
No
2p-3s
transitions.
shots at N
Fig.
spectrum
=
10
14
cm-
3
and T
The experimental spectrum is an addition of 3
2.0 x 10 1 4 cm-3 and T e
9 Three consecutive plasma
of
shots
a 1.4 keV.
with
1.4 keV.
similar
parameters
'N
=
2.9
x
Mo 32+
(2p-3s)
v0
300F
z
z
200[
+
S14+(Is-2p)
0
+ +
+0+
+
+
+
0
100 -
8
\0
__
_
I
I__
I
2
3
ELECTRON DENSITY (10+14 cm~
)
U-
I
100
100
200
200
I
/
200
204
300 -(msec)
3oo (msec)
I
"'I I""\
208
212
(msec)
Z( ~( ~o
s)'J'
N
I
.4
0
z
z
0
0
It
z
D
00
0
0
04
o
4
100
04
I
01~
On
'~
.4
~
.404
ID
c'J
~
~~
440
'
40
C,000
~
o-x ~0 00
4
D
04
4 ~
~404
4
404
q
0
+f~
In
CD
4
~
4
4oi~
o
o<
4
4
In
600
400
800
CHANNEL NUMBERS
400
0-
800
CHANNEL NUMBER
200
I
400
600
800
a
I
1i
5
C15+
C
<0
501
-j
ad
z
z
e
()
4.40
4.601
0
X(A)
-I
z
Z)
0
25
a
c
c
b
j.j
z
2p-3d
Mo (2s -3p)
8
56
5
3
4
2
7
4
I6
4.30
4.40
4
4.50
X(A)
4.60
CHANNEL NUMBER
400
800
.00
f
600
z
600
a
SI
z
m
de
0
-
k
b
0 20
n
0
Ir
9
C
I
7
z
16
Mo 2p-3d
20 15
Lii
z
24
21
201
6
w
'9
lo 2
8
I
10 12
23
13
I
9h~111
it
34
a
4.64
22
1718
I
4:68
4
4.72
25 6 27
1
28
4.76
4.80
X(A)
4.84
4.88
29
II
4.92
CHANNEL NUMBER
600
400
200
I
f
I
I
1 4+
IF II1
1000
Ii
8001*H
-J
w
9
z
z
3: 600
1-
z
0
0-
0
4001
mn
7~
qr
h..
200
a
I
Mo (2p - 3d)
20
21 24
-
Mo (2p-3s)
20
z
I-
z
19
F'
-22
-23
26
25 27
28 2930
w
L
4.62
I IIILI
4.90
,L
234
..
4.98
x(A)
5
I~
5.06
CHANNEL NUMBER
200
400
600
300
I
S800
I
I
I
11
I
s14 +
200 1-J
w
z
z
0
a
d
0
100
C
g
eh
b
If
p~
0
ill
z
Mo*(2p-3s)
w
z 2
6-
w
4
25
4.94
5.02
5.10
X(A)
5.18
S14
CHANNEL NUMBER
600
400
I
I
9
h
II
+
rF
800
I
100 I-
-J
z
z
r
z
0
0
50 F-
j
4
~
*S*
S
S
~
'*
J.l%4~
*.
*..
*
.... :~
4
~t *
I
I
Mo (2p -3s)
z
w
Z
67
2
w
8
1
9
<:
w
5.16
5.20
(A)
5.30
WAVELENGTH (A)
4.60
4.70
4.80
4.90
ALCATOR
#0730.26
60
20
#0730.27
0
00
20
-
#0730.28
*
20
200
400
600
CHANNEL NUMBERS
800