Chemistry 202-102 Test # 2 February 25, 2010 Dr. Richard Rogers
advertisement
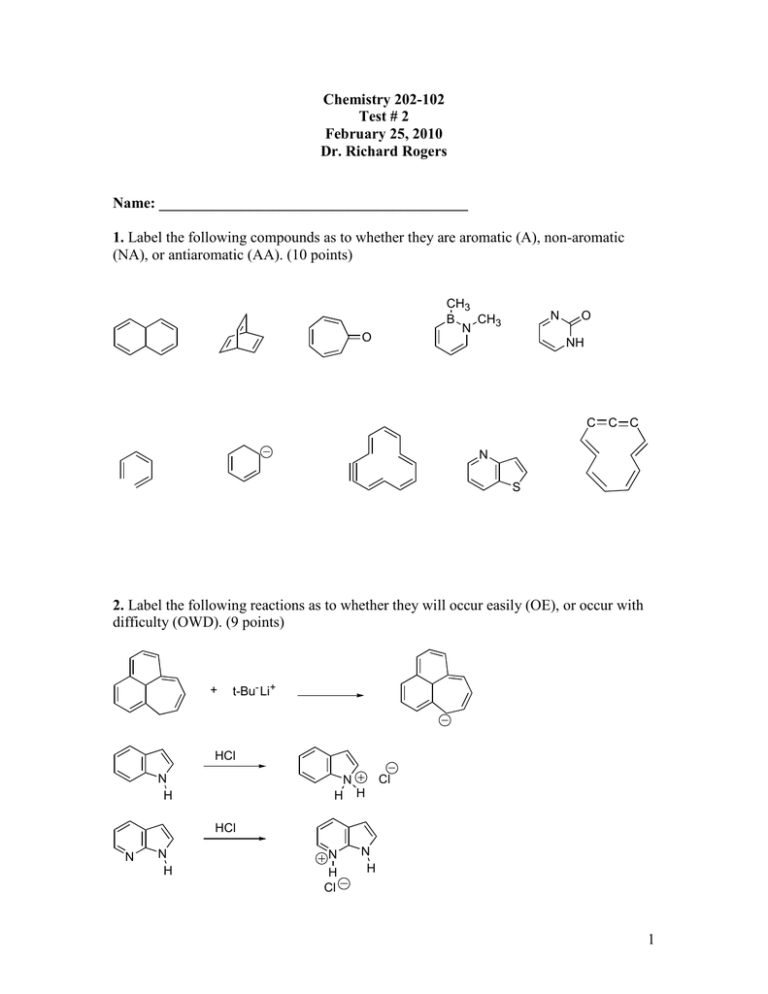
Chemistry 202-102 Test # 2 February 25, 2010 Dr. Richard Rogers Name: _________________________________________ 1. Label the following compounds as to whether they are aromatic (A), non-aromatic (NA), or antiaromatic (AA). (10 points) CH3 B CH3 N O N O NH C C C N S 2. Label the following reactions as to whether they will occur easily (OE), or occur with difficulty (OWD). (9 points) + t-Bu- Li+ HCl N H N H H Cl HCl N N H N H Cl N H 1 3. 1-azacycloheptatriene is stable at room temperature. Circle each true statement. (12 points) NH a. The compound is basic. b. The compound will add bromine across the double bonds. c. The compound will easily loose a proton to a base. d. The compound is planar. e. The nitrogen is sp3 hybridized. f. The compound is antiaromatic. g. The compound is non-planar. h. The compound is non-aromatic. 4. Draw the structure of the following compounds (6 points). a. 3-chloro-4-cyano-6-methylphenol b. 3-(N,N-dimethylamino)-5-propylnitrobenzene 5. List the following compounds according to the rate at which they will undergo bromination in the presence of an appropriate catalyst: slowest (1) to fastest (2). (6 points). NO2 CH3 OH H O Cl 2 6. For the nitration of methyl benzoate, draw the sigma complex (the reactive intermediate) which results from attack at the ortho, the meta, and the para positions. For each sigma complex, draw all appropriate resonance structures. Circle any “high energy” resonance structures. (15 points). O O + HNO3 O O + HNO3 O H2SO4 H2SO4 O + HNO3 H2SO4 3 7. Using appropriate resonance structures show atoms with high electron density for Nmethyl acetanilide (12 points). CH3 CH 3 N O 8. Draw the product and all appropriate resonance structures for the reaction shown below (6 points). H NaH H b. For the above product, how many peaks would one see in the 13C NMR (2 points)? c. For the above product, how many peaks would one see in the 1H NMR (2 points)? 4 9. Show the main product which would result from the following reactions (16 points). OH H2SO4 + Cl H2SO4 HNO3 + CH3 O + AlCl3 CH3I xs Cl AlCl3 Cl + xs O + KMnO4 NaOH H2O/100o CH3 + NBS hν Br O + NaNH2 Br 5 Cl + CH3O- Na+ Cl N CH3OH Reflux 10. Show how you would synthesize one of the following compounds starting with benzene. You may use any acylic or inorganic reagent of your choosing (10 points). OH O OH NH2 CH3 NO2 Br Cl 6