Class-X Sub
advertisement

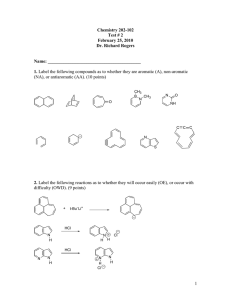
Class-X Sub-Chemistry 1)Explain the giving reaction with the example. a)Hydrogenation reaction b)Exterification reaction c)Saponification reaction 2)Explain the cleaning action of soaps. 3)A compound X is formed by the reaction of a comboxyllic acid C2H4O2 and an alcohol in presence of a few drops of conc.H2So4.The alcohol on oxidation with alkaline KMNo4 followed by acidification gives the same carboxylic acid as used in their reaction. Give the names and structure of a)Carboxyllic acid b)Alcohol c)Compound X Write the reaction also. 4)An organic acid ‘X’ is a liquid which ofter freezer during winter time in cold countries has the molecular formula C2H4O2. On warm it with ethanol in the presence of few drops of Con.H2So4 a compound ‘Y’ with a sweet smell is formed. a)Identify X and Y b)Write reactions also. Project Take five samples of commercial soaps determine their foaming capacities. (1 g soap in 100ml water. Note down time needed for , froth to disappear.)