Chemistry 202-102 Test # 1 Dr. Richard Rogers February 2, 2010
advertisement
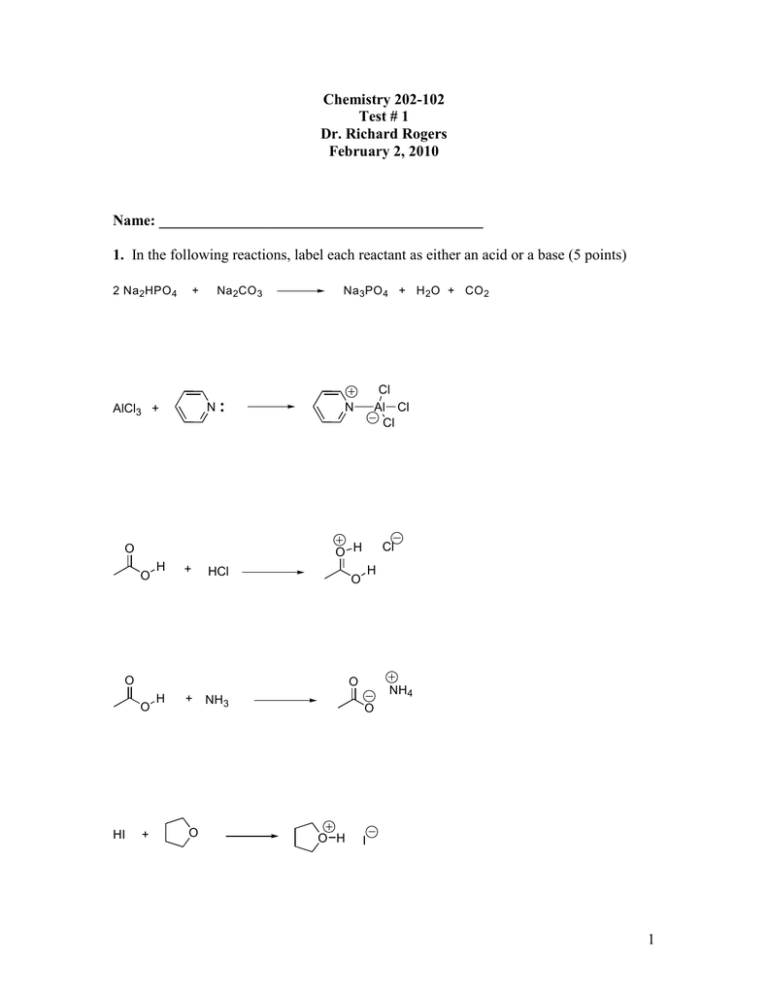
Chemistry 202-102 Test # 1 Dr. Richard Rogers February 2, 2010 Name: ___________________________________________ 1. In the following reactions, label each reactant as either an acid or a base (5 points) 2 Na 2 HPO 4 + Na 2CO 3 N AlCl3 + O O H + Na 3 PO 4 + H 2 O + CO 2 N Cl Al Cl Cl O H Cl HCl O O O HI H O + H + O NH3 NH4 O O H I 1 2. In the following reactions, label each reactant as either an electrophile or a nucleophile. Circle the actual electrophilic or nucleophilic element in each reactant (9 points). + CH3 Li O-Li+ O CH3OH + PhCH2I H Cl CH4 CH3O Li PhCH2OCH2CH3 O N + + CH3 H 3C N + HCl 3a. In the molecule shown below (3b), how many peaks will appear in the decoupled 13C nmr spectra (4 points)? 3b. In the boxes, show the number of peaks that will appear for the protons attached to the indicated carbons in the 1H nmr spectrum (6 points). Give the approximate chemical shift for the protons on the starred (*) carbon (2 points). O O * 2 4. Consider the ionic species given below. Give the hybridization of each indicated atom (7 points). C O N 4a. How many pi bonds are in this ionic species (1 point)? ______ 4b. How many p orbitals are present in the above species (1 point)? _____ 4c. The lone pair of electrons on nitrogen is in what type of orbital (1point)? _____ 4d. The lone pairs of electrons on oxygen are in what type of orbital (1 point)? _____ 5. Draw the structure of the following compounds (3 points each). 2-ethoxypent-2-ene trans-1-chloro-3-methylthiocychexane 3 6. Name the following compound (2 points). O O O O O O 7. Give the reagent necessary to carry out the followings (8 points) O-Na+ OH O O O S O O O O O O H H H3C H CH3 Cl 4 8. Define the following terms or finish the following statements (3 points each). a) sigma bond b) pi bond c) The sole requirement for resonance is the 9. In the bromination of 2-methylhex-2,4-diene (A) with NBS in the presence of light, radicals B and C are two possible intermediates. (1) Circle the allylic carbons in A (2 points). (2) Using single headed arrors to indicate electron flow, draw the resonance structures of B (4 points). (3) Draw the structure of second radical intermediate C (3 points). (4) Draw the brominated products resulting from intermediate B and circle the major product (3 points) + A C B B 5 10. Draw the products resulting from the following reactions (12 points) Ph + O CN + O O CO2CH3 + CN 11. Draw the reasonable resonance structures for the following cations and anions (10 points) O O N 6 12. Draw the structure resulting from the following reactions. Star (*) any stereocenters and label them as to whether they are “R” or “S” (10 points). OH H Ph O Cl + N H Cl NaOH O CF3 H 3C MgBr H + O H (1) ether (2) H3O+ 7