Document 11123964
advertisement

1/
~ '"'
Name:_ _ _r"
__
-1-~____
14 Oct, 2015
SC225 Quiz, Chapter 6 Prof. Urban
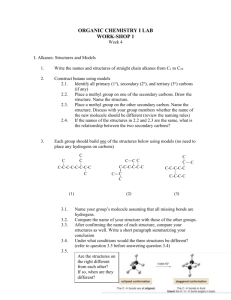
1. Consider the molecule 2,3-dimethylbutane (4 points)
2,3-dimethylbutane
a. Draw Newman projections (looking down the C2-C3 bond) ofthe three staggered
conformations. Label them A, B, and C.
A
\-\­
~*O\\-3 \,e
I+­
(~3t-/'ii;.?
2"" ,;yJ'
7~~
, ~ C tl 3
b. Which staggered conformation(s) is/are expected to be lowest in energy and why?
r
~ (~.~ ~ ~
t ,..... ~rtWf-; ........ ~
~{(.
C.
tAi.
;+
Ai
4~ ~
(1,.
c. Draw a Newman projection representing the highest-energy eclipsed conformation.
v\ \4­
\~\zA\~l
3
'1.\-;
2. Draw the chair conformation depicted in this Newman projection. (1 point)
H
H
H
H
3.
Does this alkene have the E or Z configuration?
4.
Draw the chair conformations of the molecule shown below. Indicate if they are the same in
energy or if one is lower in energy (if so, indicate which is lower in energy and why). (4 points)