Page 1 of 6 1. J. Med. Chem.
advertisement

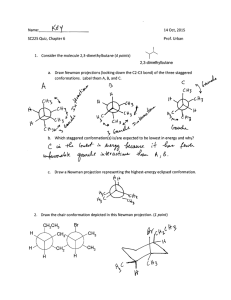
Page 1 of 6 I. Nomenclature 1. Protoapigenone, shown below, is a flavanoid compound having cytotoxic activity against several types of cancer (J. Med. Chem. 2007, 3921). Circle and identify each different functional group in protoapigenone. (5 points) 2. Provide a structure for each of the following compounds. (5 points) neopentane 3. spiro[5.4]decane Identify the correct IUPAC name for the following structure. (9 points) Page 2 of 6 II. 4. The following bile acid derivative has shown potent ability in regulating the immune system and cholesterol levels, and lipid and carbohydrate metabolism (J. Med. Chem. 2007, 4265-4268). Circle all primary (1o) carbons in the structure below. (3 points) 5. How many degrees of unsaturation are there in the structure above? (2 points) 6. An unknown compound was found to have 5 carbons, 1 ring, and only a nitrile and an ester functional group. Provide a molecular formula for the unknown compound. (3 points) Theory 1. Provide a Lewis structure for CH3NCO. (2 points) 2. How many sigma bonds are there in the structure from question #1? (2 points) 3. Fill in any missing formal charges in the structure below, and then circle all sp2 centers. (6 points) Page 3 of 6 4. Provide two other significant resonance structures for the following structure. Indicate any formal charges and circle the major contributor to the overall resonance hybrid. (8 points) 5. Draw all isomers of C3H9N and circle the structure that has the highest boiling point. (10 points) 6. State all intermolecular attractions present in pure samples of each of the following structures. (6 points) H2SO4 7. BF3 CH3CO2CH3 Translate the following line-angle structure to a condensed structure. (3 points) Page 4 of 6 8. Draw the structure represented in the Newman projection below as a wedge-dash perspective structure. (3 points) 9. Between the C2-C3 bond of butane, how much higher in energy (Kcal/mol) is the eclipsed conformation than the anti conformation? (2 points) 10. Draw the structure below in its most stable chair conformation. Label all substituents as equatorial or axial. (5 points) 11. With each structure in its most stable chair conformation, which sequence correctly ranks the compounds below in order of increasing molecular dipole moment?(3 points) 1) trans 1,4-dichlorocyclohexane 2) trans 1,3-dichlorocyclohexane 3) trans 1,2-dichlorocyclohexane a) 1<2<3 12. b) 2<3<1 c) 3<1<2 d) 3<2<1 e) 2<1<3 f) 1<3<2 Which sequence ranks the following structures in order of increasing acidity? (3 points) a) 1<2<3 b) 2<3<1 c) 3<1<2 d) 3<2<1 e) 2<1<3 f) 1<3<2 Page 5 of 6 13. Which sequence ranks the following compounds in order of increasing basicity? (3 points) a) 1<2<3 III. b) 2<3<1 c) 3<1<2 d) 3<2<1 e) 2<1<3 f) 1<3<2 14. Shown below is an energy diagram for the valance electrons of an unhybridized carbon atom. Provide a new energy diagram to represent an sp hybridized carbon atom. (2 points) 15. Draw the two molecular orbitals that would result from the combination of two atomic p orbitals. (4 points) 16. What factor of ring strain is present in cyclononane but not in cyclopropane? (2 points) IR spectroscopy 1. Indicate all functional groups represented in each IR spectra below? (9 points) Page 6 of 6 IV. Extra Credit (5 points) 1. Translate the following Newman projection to a bicyclic chair structure. Label all substituents as being equatorial or axial. You received __________ points out of 100 points possible. To check your overall performance in lecture see http://vista.weber.edu/.