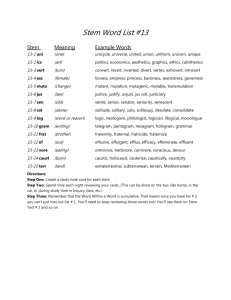
Document 10962093
advertisement

Distribution of Mutant Cells in Human Skin: Exploration of the Fetal-Juvenile Mutability Hypothesis E=# I by OF TECHNOLOGY Leslie E. Kao APR 0 2 2012 B.S. Mechanical Engineering Massachusetts Institute of Technology, 2008 UBRARIES SUBMITTED TO THE DEPARTMENT OF BIOLOGICAL ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL ENGINEERING AT THE OF TECHNOLOGY INSTITUTE MASSACHUSETTS February 2009 0 2009 Massachusetts Institute of Technology. All rights reserved Signature of Author: Signature redacted Department of Biological Engineering December 12, 2008 Certified by: Signature redacted William G. Thilly Professor f Biological Engineering f Thesis Supervisor Accepted by: Signature redacted Professor of lectrical, Mec hair, Department rodzinsky Alan cal, and Biological ngineering ommittee on Gra ate Students 2 Distribution of Mutant Cells in Human Skin: Exploration of the Fetal-Juvenile Mutability Hypothesis by Leslie E. Kao Submitted to the Department of Biological Engineering on December 12, 2008 in Partial Fulfillment of the Requirements for the Degree of Master of Science In Biological Engineering ABSTRACT The multiple "hits" carcinogenesis models are extensions of the cancer incidence theory developed by researchers from Nordling (1953), Armitage-Doll (1954 and 1957), Knudson (1971), Moolgavkar and Verzon (1979), to Moolgavkar and Knudson (1981), among others. These studies relate to the evolutionary process of normal tissue cells in an individual's organ from a normal stage to an initiated pre-neoplastic stage, and finally promoted to a neoplastic stage, resulting in tumorigenesis. The most significant impact of this type of research is to gain insight into the complex process of cancer development in humans. In the case of skin cancer, epidemiological and molecular data clearly indicate that sunlight is a carcinogen, the primary cause of skin cancer, in which forms of point mutation are associated with ultraviolet radiation. Furthermore, sunlight is attributed both as a tumor initiator and a tumor promoter by favoring the clonal expansion of p53 mutated cells. By utilizing studies of the multiple genetic hit model of oncomutation and inference that preneoplasia appears to be a clonal continuation of juvenile growth in adult tissues from which one stem cell creates an embryonic organ with lethal consequences, this thesis is devoted to the analysis of the process of skin cancer development and distribution of mutant cells in human skin, as well as the calculation of and inferences based on Gostjeva and Thilly's hypothesis on mutational clone frequency is restricted to the fetal-juvenile period. Thesis Supervisor: William G. Thilly Title: Professor of Toxicology Department of Biological Engineering 3 ACKNOWLEDGEMENTS First and foremost, I am deeply grateful to my thesis advisor, Dr. William G. Thilly, for whom I have the utmost respect and appreciation for all he has done for me. Since my undergraduate years as a UROP student, I have worked in the Biological Engineering Department under him and have benefited from his constant encouragement and academic guidance and support. I can only hope that a fraction of his profound knowledge shines through in this thesis. Thanks also go to former and current members of the Thilly Lab, who were always patient and helpful to me, providing assistance when I needed it and answering my questions. Sincere gratitude goes to Drs. Elena Gostjeva, Pablo Herrero-Jimenez, Hilary A. Coller, Xiao Li-Sucheleilki, and Hiroko Sudo, just to name a few, for providing an enormous amount of research information on my thesis topic and lending me their analytical skills to help direct my research. Reference to their publications in this thesis is the best testament of their accomplishments. I am also very grateful to Dr. Carol Warfield, my prehealth advisor of Harvard Medical School, whose stimulating input influenced the shaping of my thoughts on my research. Appreciation also goes to Rita DeMeo whose encouragement and assistance went beyond the call of duty at MIT. Finally, to my parents, Drs. George and Cassandra Kao, for their unconditional love and support. 4 LIST OF ABBREVIATIONS AK BCC Cdk CDCE CPD CS CSD dNTP CIS EPU IS KA KC LOH LOI MAMA MF(s) NBCCS NER NMSC PCR PTCH SCC SHH SPR SSCP TCR TTD TUNEL UV UVA UVB UVC UVR UVS WT XP actinic keratosis basal cell carcinoma Cyclin dependent kinase constant denaturant capillary electrophoresis cyclobutane pyrimidine dimmer Cockayne syndrome chronic sun damage denoxynucleotide triphosphate cancer in situ epidermal proliferative unit Internal standard keratoacanthoma keratinocytes loss of heterozygosity Loss of imprinting mismatch amplification mutation assay Mutant fraction(s) nevoid basal cell carcinoma syndrome nucleotide excision repair non-melanoma skin cancer Polymerase Chain Reaction patched squamous cell carcinoma sonic hedgehog sun protecting factor single strand conformation polymorphism transcription-coupled repair trichothiodystrophy terminal deoxynucleotidyltransferase-mediated dNTP nick end-labeling ultraviolet ultraviolet-A ultraviolet-B ultraviolet-C ultraviolet radiation ultraviolet sensitivity Wild-type xeroderma pigmentosum 5 LIST OF FIGURES Figure 1: Anatomy of the Skin Figure 2: United States Death Rate by Race, 2000-2004 Figure 3: United States Mortality Rate by Age at Death, 1988-1992 Figure 4-A Skin Cancer (EAM) Figure 4-B: Skin Cancer (EAF) Figure 5-A: Skin Cancer (NEAM) Figure 5-B: Skin Cancer (NEAF) Figure 6: Epidemiologic Features of Skin Cancer Figure 7: Melanoma of the Skin (SEER Incidence Rates, 1988-1992) Figure 8: Genetic and Cellular Events in the Early Development of Skin Cancer Figure 9: p53-immunopositive Clones in Whole-mount Preparations of Human Epidermis Figure 10: Increase of Clone Frequency with Sun Exposure Figure 11: Patches of p53-mutated Keratinocytes in Human Epidermis Figure 12: Increase of Clone Size with Sun Exposure Figure 13: Average Patch Size According to Sun Exposure Figure 14: Sun-Shielded Log(2) Cluster Number/Size Figure 15: Chronic Sun Exposed Log(2) Cluster Number/Size Figure 16: Intermittently Shielded Log(2) Cluster Number/Size Figure 17: Age Independence and Patch Frequency 6 Figure 18: Nuclear Fission of Bell-shaped Nuclei in the Fetal Gut Figure 19: Bell-shaped Nuclei with Higher Frequency in "Undifferentiated" Areas within Adenocarcinomas Figure 20: Pediatric Growth Charts for Male (A) and Female (B) Children of the US circa 1995 Plotted on Log-linear Coordinates 7 TABLE OF CONTENTS 1. Introduction .................................................................................... 10 1.1 Purpose and Rationale of the Study ................................................. 10 1.2 Aspects of Skin Cancer Study............................................................ 13 1.3. Scopes of Skin Cancer Study ............................................................ 14 2. On Skin Cancer: Background Review ................................................. 15 2.1 On Human Skin ......................................................................... 15 2.2 Epidemiology and Skin Cancer ........................................................ 18 2.3 Pathology/Histopathology and Skin Cancers ....................................... 25 2.3.1 Melanoma Skin Cancer (MSC) ............................................... 25 2.3.2 Nonmelanoma Skin Cancer (NMSC) .......................................... 25 2.3.2.1 Basal Cell Carcinoma (BCC) ....................................... 26 2.3.2.2 (Cutaneous) Squamous Cell Carcinoma (SCC) ............... 26 3. On Skin Cancer and Risk Factors: Literature Review ............................. 28 3.1 Environmental Causes and Ultraviolet Radiation (UVR)....................... 28 3.1.1 Ultraviolet Radiation as a Carcinogen .......................................... 28 3.1.2 Sunlight Exposure Risk Factors and Epidemiologic Features.............28 3.2 Studies of Population Migrations ................................................... 3.2.1 Ethnic Origin and Place of Residence............................... 3.2.2 Migration Origin........................ 31 ........... 32 .............. 32 3.3 Case Study of International Variations on Pigmentation and Malignant Melanoma Incidence Rates ......................................................... 32 3.4 Risk Factor of Familial Disorders, PTCH, and the Hedgehog Pathway............ 35 3.4.1 Gorlin Syndrome (NBCCS) and PTCH...................................... 35 3.4.2 PTCH and Hedgehog Pathway................................................35 3.5 Stem Cell, Hypothesis-related Studies ................................................ 8 36 4. Multiple Genetic Hit Process of Skin Cancer Development ....................... 38 4.1. Multiple Genetic Hit Theory of Cancer Development ............................. 38 4.2. Multistage Process of Skin Cancer Development ............................... 38 4.3. Sunlight, Clonal Expansion, and p53 ............................................... 40 4.3.1 On p53 Gene, p53 Mutations, Frequency, Selection, and Clonal Origin ..40 4.4. Sunlight, Clonal Expansion, Apoptosis, and Stem Cell Compartment of the 42 Epiderm is ................................................................................ 4.4.1 UV Drives Clonal Expansion of p53-mutant Keratinocytes in Normal .. 43 Skin ............................................................................ 4.4.2 Clonal Expansion involving UV-induced Physiology, not Mutation .... 43 4.4.3 Remarks on Clonal Expansion and UV-induced Apoptosis ............... 44 45 5. Application and Discussions ..................................................................... 5.1. Justification and Application Approach .......................................... 5.2. Example on "Frequent Clones of p53-mutated Keratinocytes in Normal Human .. 47 Skin" by Jonason et al............................................................. 5.2.1 5.3. 45 Materials and Methods .................................................... 47 p53-Mutant Clones, Sun Exposure, Clone Number, Size, and Frequency .... 47 5.3.1 p53-Mutant Clones.................................................. 5.3.2 Number and Size of Cells in p53-Mutant Clones .................. ......... 47 ....... 51 5.4. Patch Frequency is Independence of Age ........................................... 56 5.5. Inference to Fetal Juvenile Mutability Hypothesis and Discussion.............. 59 6. Summary of Interpretations and Conclusion ............................................. R eferences ........................................................................................... 9 66 69 Chapter 1 Introduction 1.1. Purpose and Rationale of the Study The reasons for and causes of cancer have flummoxed scientists for hundreds of years. The current theory is that of a multistage genetic hit model of cancer that predicts that normal individuals have stable populations of pre-cancerous mutant cells awaiting further genetic hits. Based upon previous observations and calculations, the team of Gostjeva and Thilly expanded upon this multistage model in their fetal-juvenile stem cell initiation hypothesis by identifying the at risk cells and a time frame in which the initial mutations would occur (1-2). Observation of historical and present day age-specific colorectal rates, and direct measurements of genetic changes in human tissue as a function of age led Thilly and Herrero-Jimenez et al. to hypothesize that initiated stem cells grow at near juvenile rates to form a preneoplastic colony [3-4]. Further initiation would occur within this colony from which would emerge a neoplastic stem cell with near fetal growth rates to form a lethal tumor. Specifically, in the case of colon cancer, the calculated growth of the preneoplasia was equal to the growth rate of the juvenile colon, suggesting that tumor initiation blocked the developmental step that transformed a juvenile stem cell into an adult maintenance stem cell. Tumor promotion could be achieved by transformation of an initiated stem cell 10 within the slow growing precancerous colonic polyps into a fetal stem cell with a rapid net growth and the capability to differentiate [1]. While the multistage model of cancer formation had been uncovered, the question of identifying and visualizing the specific stem cells at risk still remained. Through the reanalysis of fetal and adult colonic tissue with a novel histological preparation method, Gostjeva discovered that fetal and neoplastic tissues share a set of cells with unique non-spherical nuclear morphotypes which appear to cooperate in creating the elements of the fetal organ, preneopastic and neoplastic lesions. Most tellingly, examination of fetal tissue showed the presence of tubular syncytia containing openedmouthed, bell-shaped nuclei that underwent amitotic symmetric and asymmetric nuclear fissions, the later of which gives rise to seven other nuclear morphotypes. These other nuclear morphotypes subsequently divide extra-syncytially by mitoses to form clonal populations of cells with identical nuclear morphotypes in embryos, adenomas, adenocarcinomas and metastases. Bell-shaped nuclei have been found in, and thus appear to be responsible for both net growth and differentiation in the embryonic gut, adenomas and adenocarcinomas, and fulfill the requirements for post-embryonic stem cells in colon organogenesis and carcinogenesis [1]. Gostjeva and Thilly inferred that these bell-shaped nuclei represent the "metakaryotic" stem cells that drive net growth and differentiation in fetal, neonatal and juvenile development and in colonic adenomas, adenocarcinomas and metastases [2]. Further support for the fetal-juvenile stem cell initiation hypothesis came in the form of measurements taken from the lung by Sudo et al. in which it was found that 11 smoking and aging have no effect on the measured point mutant fractions nor on their numerical distributions in the adult lung epithelium. This lack of increase in point mutation fractions lead to the conclusion that the point mutations must have occurred before maturity. These mutant fractions are constant with age in adults because mutations occur much less frequently in adult maintenance stem cells than in pre-adult stem cells involved in fetal and juvenile growth. Furthermore, Sudo et al. proposed that the measured mutations arose during an exponential increase in stem cell number during human organ growth and development, possibly within Gostjeva's metakaryotic cells [5]. Concurrent with the research done on the genetic factors leading to cancer, much study has been done examining the possible effect of environmental factors on the progression of carcinomas. Epidemiological analyses of age-specific cancer rates show large historical increases. Since inherited genetic conditions could not make an appreciable change in only a few generations, environmental factors had to be the catalyst. Supported by the fact that the large increases in age-specific mortality rates for several forms of cancer occur concurrently with the population shifts from the agrarian to urban, the abundance of potential human mutagens in our daily environment and the resulting products of chemical reactions between environmental chemicals and DNA or proteins in human tissues can be pointed to as the catalyst for the historical increase in cancer rates [6]. This thesis will rely upon skin cancer to test the validity of both the genetic and environmental theories. 12 1.2. Aspects of Skin Cancer Study By reanalyzing data from Brash, Ponten and Jonason et al.'s research and findings [7], and applying current theoretical hypotheses, this thesis is designed to investigate the mechanism and development of skin cancer [7]. Two aspects will be emphasized: First. Sunlight is an environmental carcinogen that acts as both a tumor inducer and promoter. Skin cancer is the only cancer that provides a link between skin cell mutations and sunlight, in which forms of point mutations are associated with ultraviolet radiation (UVR) [6]. Brash et al. stated that sunlight is a carcinogen - an "initiator" which produces mutations and leaves its molecular footprint, or "signature," in the DNA C-+T transitions at dipyrimidine sites predominate, including CC-+TT double-base mutations [10]. In addition, in their study of frequent clones of p53-mutated keratinocytes in normal human skin, Jonason et al. stated that normal human skin contains multiple colonies of keratinocytes that stain for aberrant p53 protein [8]. By observing that higher frequency and larger mutant patch sizes occur in sun-exposed skin, they concluded that sunlight acted as a tumor inducer as well as a tumor promoter, providing selective growth advantage to both endogenous and exogenous mutants [8-9]. Second. Distribution of mutant cells in human skin and stem cell mutation limited to the fetal-juvenile period hypothesis: Their study also observed that p53 patch frequency is independent of age based upon the finding that substantial mutagenesis seems to occur during childhood and much 13 less during adulthood, if any [8]. Similar epidemiological evidence relating to sunlight exposure before age 18, as noted by Marks, Kricker, English, Leffell, Ziegler, Ponten, Ren, and Brash et al. [10-24] closely parallels this thesis' second focus to apply the GostjevaThilly hypothesis in which skin cancer in adults occurs at constant rates in pre-adult stem cells involved in fetal and juvenile growth as implied by the fetal-juvenile mutator stem cell hypothesis [1-2]. 1.3. Scopes of Skin Cancer Study Accordingly, emphasis for this thesis is twofold: The first part focuses on human skin, skin precursors, skin cancer types, and offers a review of relevant research and scholarship. Subsequently, by utilizing studies of the multiple genetic hit model of oncomutation in developing fetal juvenile tissues, the second part of this thesis is devoted to the analysis of the process of skin cancer development, p53, distribution of mutant cells in human skin, as well as the calculation of and inferences based on Gostjeva-Thilly's fetal-juvenile hypothesis. 14 IBM- Chapter 2 On Skin Cancer: Background Review 2.1 On Human Skin Consisting of approximately one sixth of the human body's total weight, the skin is the largest human organ. In its physiological anatomy, the skin consists of several layers: the epidermis is the outermost layer with an inner dermis layer in conjunction with subcutaneous tissues. See Figure 1: /Epidermis Dermis Squarnous cells Basal cells Melanocyte Figure 1: Anatomy of the Skin Retrieved from: http://www.cancer.gov/cancertopics/wyntk/skin/page2 15 Up to 90% of the cells in the epidermis are keratinocytes (KCs), which function as a barrier preventing the invasion of harmful substances and minimizing water loss from the body through the epidermis. The other portion of the epidermal cells are melanocytes, called stratum basale, which produce melanin, the protein that adds pigment to skin keratinocytes and thus protects the human body from ultraviolet rays (UVR). In general, the skin darkening process due to sunlight exposure results from the processed movement of existing melanin into keratinocytes, along with the increase of melanin by the melanocyte [7. As indicated in Figure 1, the dermis, or the "true skin," is located under the epidermis. The dermis is composed of elastic materials, water, and collagen. Embedded in this layer are the blood vessels, lymph vessels, and glands. While certain glands produce sweat to cool the body, other glands make sebum to keep the skin from drying out. Both sweat and sebum reach the skin surface through small openings called pores. The epidermis is comprised of five distinct layers of stratified keratinized epithelium that represent a progressive maturation and differentiation of the keratinocytes. The deepest layer, the stratum germinativum or stratum basale, is a single layer of columnar basal cells that undergo mitosis to produce new keratinocytes that move to the skin surface, replacing cells lost during normal skin shedding. The basal cells remain stationary in the statum germinativum. With an observed pathology curiously similar to Gostjeva's bell-shaped nuclei, columnar cells possess ovoid or "indented" nuclei with one or two nucleoli embedded in 16 euchromatin surrounded by a thin rim of heterochromatin. Occasionally two or three rows of these cells may be present with the appearance of smaller oval daughter cells through mitosis. The mitotic index is approximately 2 cells for every 1000 cells. The next deepest layer, the stratum spinosum, is formed from the progeny of the basal cell layer as they move outward. Two to four layers thick, the "pickle" cells of the stratum spinosum differentiate as they move outward. The stratum granulosum consists of granular cells that are the most differentiated cells of the living skin. While some cells in this layer loose cytoplasm and DNA structure, others continue to synthesize keratin. Mostly confined to the palms of the hand and the soles of the feet, the stratum lucidum lies adjacent to the stratum granulosum and appears as a thin, transparent layer that retains some function of living cells from deeper layers but otherwise resembles cells of the stratum corneum. This top or surface layer is composed of dead keratinized cells ranging from 15 to 25 to 100 layers thick in some regions. The epidermis is maintained through the proliferation of keratinocytes, which retain the ability to self-renew throughout one's life time and produce daughter cells, which undergo terminal differentiation and death [7, 25-26]. More explicitly, the epidermis is comprised of an epidermal proliferative turnover unit in which a skin cell residing in the basal layer populates a defined region of overlying skin [27]. Human skin is a fine mosaic of "tiles" or clusters of clonally derived cells consisting of around 20 to 350 basal keratinocytes [28]. The significance of keratinocytes lies in its continuous proliferation, differentiation, and apoptosis via its stem cells, transient-amplifying cells, and committed cells [29]. In 17 comparison, columnar basal cells represent around 10% of the keratinocyte cell population, generating daughter cells from mitosis which are either stem cells or transient-amplifying cells. Transient-amplified cells represent around 50% of the basal cell population, producing daughter cells that lead to stages of terminal differentiation and death [29]. 2.2. Epidemiology and Skin Cancer Skin cancers are categorized according to their cancerous cell of origin: In melanoma skin cancer (MSC) -- melanoma from melanocytes or melanocyte precursors, and nonmelanoma skin cancer (NMSC) -- around 80% of the tumors are basal cell carcinomas and 20% are squamous cell carcinomas. The two common types of cancer under the nonmelanoma skin cancer category are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [30]. According to the National Cancer Institute, skin cancer, the broad category for cancers arising from the different cells that make up cancers arising from the different cells that make up the skin, is the most commonly diagnosed cancer type in the United States. An estimated 59,940 new cases (33,910 men and 26,030 women) were diagnosed with melanoma of the skin in 2007 - 8,110 of which are predicted to die of this type of cancer. In 2007 in the category of nonmelanoma skin cancer (NMSC), more than 1 million new American cases were diagnosed (www.cancer.gov/cancertopics/wyntk/skin). Broken down by race, Figure 2 below includes death rates due to the melanoma skin cancer between 2000 and 2004. The age-adjusted death rate was 2.6 per 100,000 men and women per year -- white males and females constitute the highest death rate with 4.3 18 deaths per 100,000 white men, and 2.0 deaths per 100,000 white women. Males and females of Asian and Pacific Islander decent constitute the lowest death rate at 0.4 per 100,000 for Asian/Pacific Islander males, and 0.3 per 100,000 for Asian/Pacific Islander women: Figure 2: Death Rates by Race Female Race/Ethnicity Male All Races 3.9 per 100,000 men 1.7 per 100,000 women White 4.3 per 100,000 men [2.0 per 100,000 women Black 0.5 per 100,000 men [0.4 per 100,000 women Asian/Pacific Islander 0.4 per 100,000 men [0.3 per 100,000 women American Indian/Alaska Native a 1.3 per 100,000 men [0.7 per 100,000 women Hispanic b 0.9 per 100,000 men 0.6 per 100,000 women Retrieved from http://www.cancer.gov/cancertopics/wyntk/skin/page2 Figure 3 below offers mortality rates in the United States due for melanoma of the skin between 1988 and 1992 (from National Cancer Institute, SEER Program, ibid.): 19 MELANOMA OF THE SKIN United States MORTALITY Rates by Age at Death, 1988-1992 WOMEN MEN * * ALASKA NATIVE 7F =-IN N W, VEXC; AMERICAN NDIAN (NEW VEXIOC": BLACK *2 C-IINESE * .-Ilk FILl- '.:: * A FIL!P NC AGE 30-54 JAD'ANESE NA VREA IET 3 I1 .2 VIETNAMESE ..,F TE N"A F S=A'-IC 4TCTALi 4: tE HIS'AN C J N N-H ISPAN ;C NCNHSPAN 3 ALA:3'A NA C'S 1C 2C 3C 4C E5 ,O 70 SC 10 20 3e: 40 3C 60 70 30 * * A-ASKANATIE AMERICAN ND AN NEW MEXICO' 1.:. Ii BLACK * * Ci- NESE FILIFNC AGE 55-69 JAPANESE 4 Ci KOREAN N:A VI TNA-IE NA - ' F-55II FAAIN '>L FESFNIC I HS--C C i:-AN A--AN,TI'. 24 I'2 I.3 AN.r-.TOTAL : HIS -- -1SPANIC f- ' -N--I-PANI' 20 ' 30 40 50 53 70 s 3 * * O 0 AMERICAN ND.AN *NEW MEXICO: AN E BLACK *9 *. C-INESE * I..-'- i * FILIPINC AGE 70+ JA'ANESE KOREAN N. A *7 VIETNAMESE * 4 -PA 3.1 ,%HITE '3.4 -I'A-a 'HTE C TI: TAL -ISF FSFANIC TCTAJ --A ANIC TE HISPAN 27 C WHITE N01-- SFANIC NC'N-HISPANSIC 10 NOTE: Razes are "averageannua 'per 1 1030,0 populat'on, age-adjsted to 1970 20 52 CK 5 U.S. standard, N/A =data unaivailable:*= fewer than 25 deaths. The following skin cancer age-year-specific and birth-year-specific mortality data in Figure 4-A & B and Figure 5-A & B collected and organized by Herrero-Jimenez of the Thilly group, are available at http://epidemiology.mit.edu [3-4]. These are quantitative analyses of public health data for skin cancer in the US were maintained by the US Census Bureau (1870-1935) and later by the US Public Health Service (1936-1997): Figure 4-A: Skin Cancer Skin Cancer (EAM) . 10 1800 ... ... 10.. 10 --- - ... ..10...- 100 1810 1820 .-. 1830 1840 + 1850 -.-- 1860 1870 -. 1880 .--. 1890 1900 --A - ...... . -A 1910 - - -e1930 e1920 - . 10-- 1940 --1950 -- 1 i--1970 S20 -- : 1960- 1980 40 60 80 Age (yeais) 100 120 Figure 4-A: This is skin cancer age-year-specific and birth-year-specific mortality rates From http://wgt5.mit.edu. EAM stands for European-American males. 21 Figure 4-B: Skin Cancer Skin Cancer (EAF) ............................................... ................ --------------- ------------------------------........ ................ ----------................ ------------------------------------------................................ --------- ---------------- -----................................................... 10 ........................................... ........... ------------- :------ I--------- :-------............................................................ .............................................................. ................,---------------- ------ -------- ----------....... .. ............ .. ..... ;11 ............................................... 101 .......................... - - .......................................... ............. .. ......................... ----------I ................:_ ................................. ............... ... . ..... ................. ........... .................:. - -,- - x .. _': ::::::: ............................... ----------------------------------------------- --------------------::-: -: -:-::- -: -: -:-: -:-:-: -: -:-: -:.......... ------10 1------------------------------------------------------------------..... :......... ------- ---------------------------------------------------------................ ................ . - 0'. t---------------............ -------- -------------------------------------- .............. . ............................. 10-1 .......... ...... ......... -----------------::: .............. .............. : .................................................. ................ .............................. .................................... ............... :--------------- 1: ---------------- :--------------.:.............................................. ........................ 41: 7' 4 . . ......................... ........................................ I ... ........................................ --------------- ............... ........................:............... .................................. -------------------------------------------------------------------------------_-------------- ---------------- ------------- _ ............... -------- -- 10-10 20 40 60 80 160 1800 11 88 21 00 18 30 1840 1850 18 6 0 18 70 .1880 1890 '190 0 191 0 1920 1930 1 94 0 1 9 50 1960 1 9 70 1980 __Jj 120 Age (years) Figure 4-B: This is skin cancer age-year- specific and birth-year- specific mortality rates. From http://wgt5.mit.edu. EAF stands for European-American females. 22 Figure 5-A: Skin Cancer Skin Cancer (NEAM) to-: ............... ..... ............................... ............................ ................... ........... ......... ................................ ---------------- ............... ............... .......................................................... ....... ......................... ............... ...... ...... --------- - ....... - .1- ............... .... ............ .................... 182 0 1830 1 8 40 to, --------- ............... ............ ............. . ...... ................................................. 1 85 0 ........ .... ---. ...... --.................... .................. ................ ............... ............ .. :: .................... ...................................... 1860 ......................... .......... - ...... .................... ------1870 1880 ......... too ........... ::::' :::Mw:MMw:Mww... . ................... 1890 ..................... - ----------- -------------- ....... *................... ----------------......... ......I ........ ----------- ..... .................. 1 900 .............. ............................ A 1-1 ......................................................... ..................... ......... 19 1 0 ........... 1 92 0 ---------------- ---------------- ................... ........... ............... .. 193 0 to, .......................................................... .......................... ..................................... 1940 .................................. .............. :........ ....... ...... ........................ . : -----------..... **-,* ....... T '7 ....................................... 1 9 50 . ................................ ........................................................ 1960 1970 to-, ................... ----------- .................................... 19 8 0 ------------------- ----------------........................................... .............................................................. ... 4 ......I .............. :-*-,**-,* ....... :---------------- :........ **......... .............................................................. .......... I.............. .......................................... ................................ ------------- 10"'I 0 20 40 60 80 Ago, (vears) 100 120 Figure 5-A : Skin cancer age-year- specific and birth-year-specific mortality rates From http://wgt5.mit.edu. NEAM stands for Non-European-American males. 23 Figure 5-B: Skin Cancer Skin Cancer (NEAF) ................ 4 ------.......................... .......... ........................ ......... ......................... .........:................ _ _ _ ....... -------------- _----------............... : ---------------: ........ I .............................. ....................................... .......................................... ........................... ......... 1820 .......... ..... ....... ....... ................ 1830 1840 to ................. ........................... 18 5 0 --------------------------------------. ................. ............ ------ ........ ............ . ........................................... ... 1860 . .... ---. ................. -----------------18 7 0 ................ ............... ....... ......... ........ . 1880 CT to . ......................... .............. 189 0 . . ........ ............................ ................... ........ .................... ....................... 1900 ............................................... ............... .. ......................... ......... 19 1 0 ........... ---------------- --------------192 0 . to ....... V: ........... Ar A 1930 ............................................. ........................................... *.............. 19 4 0 .................. ............... ..................................................... ........ . 1 9 50 ............................................. ----------......... 1960 -----------------*1970 ................ ................ ............... 10::::: I_ ::: 1980 ..... .................. .... ............ .................................................................... ............... ..........: -------------- : ............. ................................................ ---------- ------------ ...... ........................................... ----- --------- -................I -------- -- ------ ---------- 11- ------- ------10 4 20 40 60 80 Age (years) 100 120 Figure 5-B: Skin cancer age-year-specific and b irth-year- specific mortality rates From http://wgt5.mit.edu. NEAF stands for Non-European American females. 24 2.3. Pathology/Histopathology and Skin Cancer 2.3.1 Melanoma Skin Cancer (MSC) Melanoma skin cancer (MSC), also known as malignant melanoma, is a malignancy which originates from melanocytes, the pigment-producing cells in the skin [31]. Early melanomas are seen clinically as flat, pigmented macules that mostly follow the ABCD rule - asymmetry, boarder irregularity, color variegation and lesional diameter > 6 mm [32-33]. Histopathologically, neoplastic melanocytes of melanoma in situ are located within the epidermis, where focal proliferation in the basal layer occurs in early stages [33]. Due to the fact that the nuclei of these cells at this initial stage may appear normal, accurate histopathological diagnosis may be impossible. This type of diagnosis only becomes possible after some time has passed, during which solitiary melanocytes extend horizontally along the basal layer and develop atypical nuclei. These atypical melanocytes ascend to form poorly demarcated nests of variable sizes and shapes [33]. 2.3.2 Nonmelanoma Skin Cancer (NMSC) Nonmelanoma skin cancer is most common among white populations (see Figure 2). Around 80% of the tumors are basal cell carcinomas (BCCs), the remaining 20% can be categorized as squamous cell carcinoma (SCC) [50]. Putative precursor lesions (e.g. actinic keratoses) to SCCs are very common. As shown in Figure 2, NMSC is the most common human cancer in predominantly Caucasian populations of certain areas of the world - such as the high percentage presented with NMSC each year in the United States and Australia [11, 34]. Marks' 1989 and 1990 reports state that over half the population 25 above the age of 40 has NMSCs or cognate lesions in Australia due to high ambient ultraviolet radiation and a predominantly white population [11-12]. 2.3.2.1 Basal Cell Carcinoma (BCC) A typical BCC arises from the basal cells that form a border between the dermis and the epidermis. BCCs occur as small, shiny pink or pearly lumps with an ulcerated center and are most commonly found in sun-exposed skin, such as one's face, and less commonly on the upper trunk or non-exposed place. From a histological point of view, the tumors display downgrowths of keratinocytes from the epidermis lined by palisades of cells resembling basal cells [30]. Since BCCs do not metastasize, they are almost always harmless and can be cured at any stage if excised completely via surgical therapy or radiotherapy, which explains the low death-rate for BCCs [30]. 2.3.2.2 Squamous Cell Carcinoma (SCC) Squamous cell carcinomas (SCCs) can be defined as invasive tumors with the ability to metastasize [30]. Originating in sun-exposed skin, SCCs grow quicker than BCCs, and create more irregular keratotic lesion with ulceration. In SCCs, metastasis rates are between 0.1% and 4%, and, the overall mortality is low [30]. Both cutaneous malignant melanoma and squamous cell carcinomas arise from the squamous cells in the epidermis and have an initial progression stage in which proliferation of the neoplastic cells are mostly confined to the epidermis [33]. 26 Early cutaneous squamous cell carcinoma (SCC) occurs in several different ways involving multiple lesions that are clinically characterized according to the carcinogenic stimuli which cause the epidermal keratinocyte transformation, including solar keratosis, thermal keratosis, arsenic keratosis, radiation keratosis Bowen's disease, bowenoid papulosis, and leukoplakia [33]. These lesions are considered precursors to cutaneous SCC because they are: (i) biologically benign with only a few progressing to invasive SCC; (ii) histopathologically atypical - made up of large, pleomorphic nuclei; (iii) cytologically indistinguishable from the atypical keratinocytes of invasive SCC; and (iv) clinically as well as histopathologically, no well-defined boundary exists between precursor lesions and lesions of invasive SCC. If left untreated, precursor lesions have the potential to become malignant, metastasizing deeper tissues [33]. Considered the most commonly observed form of early cutaneous SCC, solar keratoses usually occur as multiple lesions, <1 mm in diameter, and covered by adherent scales. Lesions are more often found in the sun-exposed skin of fair-skinned elderly persons with the atypical solar keratinocytes in the basal layer of the epidermis. Later proliferation occurs in the mid- to upper epidermis in which the keratinocytes lose polarity and arrange themselves in a disorderly fashion [33]. 27 Chapter 3 On Skin Cancer and Risk Factors: Literature Review 3.1. Environmental Causes and Ultraviolet Radiation (UVR) Epidemiological and molecular data clearly indicate that sunlight is a carcinogen to which everyone is exposed [10, 13-14]. Sun-exposure is also attributed as the primary risk factor and main environmental cause of skin cancer [10, 13-17], which causes DNA damage, sunburn, mutation-associated inactivation of the p53 tumor suppressor gene and clonal expansion [10, 13, 17, 19, 35]. 3.1.1 Ultraviolet Radiation as a Carcinogen The ultraviolet radiation (UVR) contains three spectra that are distinguished by their respective wavelengths: UVA containing wavelengths from 320 nm to 400 nm; UVB, from 290 nm to 320 nm; and UVC, from 100 nm to 290 nm [10]. While UVC is filtered out by the ozone layer, both UVA and UVB can reach the surface of the earth. UVA, which constitute 95% of surface UVR rays, is absorbed by the dermis and cause the skin photoaging of the skin, while UVB is absorbed by the epidermis and has been demonstrated to cause DNA damage [10, 18, 29]. 28 Ultraviolet light produces mutations and leaves marks in the DNA. UVR induced mutations are C -> T transitions, occurring at dipyrimidine sites, and include CC -> TT double-base mutations (10). 3.1.2 Sunlight Exposure Risk Factors and Epidemiologic Features Six categories based on higher frequencies that indicate sunlight as the main risk factor for human skin cancer have been identified by English et al. [15]. These six are: (1) people living in areas of high ambient solar irradiance; (2) people with sun-sensitivity; (3) people with high sun-exposure; (4) people who expose more body area to the sun; (5) people with benign sun-related skin conditions; and (6) people less damaged by more skin protection against the sun [15]. The epidemiology of skin cancers includes melanoma and nonmelanocytic skin cancers (squamous cell carcinoma [SCC], and basal cell carcinoma [BCC]), as well as melanoma of the eye [15]. English et al. divide these cancer risk factors into three distinct categories for further analytical studies: (1) Descriptive Category (including describing people's ethnic origin, place of residence, migration, occupation, and anatomic site); (2) Analytic Category (including analyzing the sensitivity of people's skin to sunlight, ambient sunlight at places of residence, exposure of the skin to sunlight [total exposure, occupational exposure, non-occupational exposure, and sunburn], other sun-related skin damage, and protection against sunlight); and (3) Molecular Effects Category (such as effects of DNA repair deficiency, and mutations in tumors characteristic of UV radiation) 29 [15]. The summarization of these epidemiologic features highlighting sunlight's role in causing melanoma, BCC, and SCC cancers as reflected in Figure 6 below: Figure 6: BCC SCC yes yes yes no yes yes yes yes no yes yes yes yes no yes yes yes yes yes yes yes no no yes yes yes no no no yes yes yes no yes yes no yes yes no yes yes yes yes yes yes Melanoma Epidemiologic Features 1. Descriptive Category: Ethnic origin Place of residence Migration Occupation Anatomic site 2. Analytic Category: Sensitivity of skin to sunlight Ambient sunlightat residenceplaces Exposure to sunlight: Total exposure Occupationalexposure Non-occupationalexposure Sunburn Other sun-relatedskin damage Protection againstsunlight 2. Molecular effects of ultraviolet radiation: Effects of DNA repairdeficiency Mutations in tumors characteristicof UV radiation Summarization of D. R. English's Epidemiologic Features of Skin Cancers: Melanoma, BCC, and SCC [15] 30 The above chart reveals that various details related to sunlight are indicated as potential causes of skin cancer. However, there are some differences among melanoma, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC). For instance, in the second category, both melanoma and basal cell carcinoma (BCC) are implicated more with non-occupational exposure than occupational exposure to sunlight. Squamous cell carcinoma (SCC), on the contrary, is related more to occupational and non-occupational exposure to sunlight [15]. 3.2. Studies of Population Migrations Various studies conducted by English, Leffell, Kricker, and Marks et aL. on the migrations of white Australians have illustrated the distribution of epidemiological features [11-17, 20-21]. 3.2.1 Ethnic Origin and Place of Residence Early epidemiologic studies conducted as far back as 50 years ago, show that white Australians - who predominantly descended from the fair skinned, light haired populations of the British Isles - experience the highest rates of all types of skin cancer in the world. Their genetic relatives, living under cloudier conditions were more fortunate, as were the darker skinned Australian Aborigines with whom they shared an environment [16]. In the United States, the incidence of skin cancer is about 20-fold higher in Whites of European origin than in Blacks [15]. Melanomas and NMSCs are the densest in chronically sunexposed sites (head and neck) and the least dense in rarely exposed sites (buttocks and 31 abdomen). As a result, fair skin and strong sun exposure have been identified as strong risk factors for skin cancer [15]. 3.2.2 Migration Origin In addition, studies that when people of European origin migrate from places of low incidence melanoma and low ambient sunlight to places of high incidence and high ambient sunlight, subsequent rates of melanoma were higher than those of their home country. Kricker [20], Marks [21], and English [15] further noted that people who had migrated from the lower ambient sunlight of England before the age of 18 to Australia with its high ambient sunlight acquired the higher Australian incidence of NMSCs. However, if migration occurred after the age of 18, they retained the risk of their country of origin [3334]. These findings suggest that Australian skin cancer patients receive a critically high dose of sunlight years before the appearance of tumors, which typically arise in patients, between the age of 50-70 [7, 16]. This aspect will be discussed further in the next two chapters. 3.3 Case Study of International Variations on Pigmentation and Malignant Melanoma Incidence Rates There is a clear correlation between malignant melanoma incidence rates and racial composition, and the intensity of sunlight exposure in different geographic areas. Rates are low in races with the most skin pigmentation, such as blacks and Asians, and are high in whites. Among whites, as indicated by Marks et al., incidence rates are about four times higher in Australia and New Zealand than in England and Scotland [21]. According to the 32 SEER of the National Cancer Institute, among white populations in the United States, the risk for malignant melanoma is highest for fair-skinned people, while Asian, black, and Hispanic people are among the lowest groups. The differences in pigmentary characteristics between different populations are largely attributed as one of the risk factors that lead to malignant melanoma incidence rates, as indicated in Figure 7 below (http://www.cancer.gov/cancertopics/wyntk/skin): 33 Figure 7: MELANOMA OF THE SKIN SEER INCIDENCE Rates, 1988-1992 WOMENN ALASKA NA~ VE AMERICAN INDIAN (NW AEXCCC AMERICAN NDIAN NEWV MEXIC BLAC K * SLACK * NATIVE * ALAS<A ___ * MEN C-INESE FILI C-NESE N' FILiIN -AWAIIAN ,AFA.NES- JA'ANESE K,-REAN <OREAN VIETNAMESE 4,5 -7- :T:~TAL:. HISPANIC Ug U 2.2CC C -IS-'AN NVN-HIS-ANC ' 1: 10 I WHITE C 3.2 TC'A-j -ISPANIC WHITE ' -ISPANIC 9 'VIE~NAVESE W- ~E WCHI C) -AWAJ AN 3. WHITE NCN--ISFANIC 0 1r 20 15 20 United States MORT ALITY Rates, 1988-1992 WOMEN MEN * ALASKA NATIVE * ALASKA NATIVE A*VERICAN NDIAN NEW MEXIC-i \DIAN MEXI CC A-MnICAN :NEV EACK BLACK C-INESE CHINESE F L FILIFINO 'INO HAWA IAN -A'IAAN FAPANE SE J'ANESE <CDREAN NWA KCR=LAN NA V AT\AMESE NeA VIETNAMESE NA WH~E .7 WH THE lIE H SFANIC 7:~MTAL WHI~E ,ISPANIC WHIE HISPANiC WHI~E WHITE N C N-HISPANIC NK)N--1S'ANIC 2 197$ S. standaro: ~K)TAL; -1S='ANC NOTE: Ra:es a-e * = It. National CancerInstitute WA 20 'average ann-al' per 'CO.EC ptoa:io' rate 'a: calca:e whee fewer tha' 2$ cases. 0- age-adjusIed O NsA 85 ' =inbrmation no: ava able: S EER Program 34 3.4. 3.4.1 Risk Factor of Familial Disorders, PTCH and the Hedgehog Pathway Gorlin Syndrome (NBCCS) and PTCH: The most common familial disorder is the nevoid basal cell carcinoma syndrome (NBCCS, Gorlin syndrome), which roughly constitutes less than 1% of all cases of skin cancer [30]. In Gorlin syndrome, BCC is the most prevalent tumor which features an autosomal dominant disorder characterized by multiple BCCs with onset at an early age and with many forms of congenital malformations, such as keratocysts of the jaw. Brash et al. stated that patients with Gorlin syndrome usually inherit a point mutation and loss of the second allele in a single cell that causes either a tumor or the aforementioned congenital malformation [18]. The gene responsible for NBCCS was mapped by groups to 9q22-31 and identified as the patched gene, PTCH, which is a human homologue of the Drosophilagene patched (PTC) [18, 29-30, 32]. Brash et al. explained further that PTCH is mutated in about 90% of BCCs, and that inherited mutations in the gene cause Gorlin syndrome [18]. 3.4.2 PTCH and Hedgehog Pathway Brash and Ponten further explained that the sequencing of BCCs indicated that nearly all BCCs contain PTCH mutations. PTCH encodes for a large glycoprotein with 12 membrane-spanning domains and two large extra-cellular loops. Previous Drosophila studies stated that this glycoprotein is part of the hedgehog signaling pathway, which played an important part in early patterning determination and cell fate in which PTCH participates in a complex with a membrane protein, smo. Uncoupled, smoothened appeared 35 to be active, while the complex with patched inhibited smo activity through the binding of the secreted hedgehog signaling protein. Inhibition of smo induces transcription of smoothened downstream target genes. In its inactive form on which the hedgehog pathway was placed, patched would be the most visible. An inactivating mutation in patched would lead to an increase in its transcription, as evidenced in BCC tumors [7]. Gailani et al. showed that expression of PTCH was barely detectable in the epidermis, while readily detectible in tumors, suggesting upregulation of the gene only after both alleles of PTCH were mutated [36]. Brash and Ponten suggested that PTCH inactivation resulted in skin tumors through the resulting upregulation of other hedgehog target genes, specifically sonic hedgehog (SHH), the most common vertebrate homologue of hedgehog required for correct patterning of the neural tube and somites, and for anterior/posterior positioning of the limb bud. Transgenic mice with overexpression of SHH have been shown to develop many features of Gorlin syndrome [7]. Gailani et al. also reported that the loss of heterozygosity (LOH) data had been located in more than half of sporadic BCCs [37]. Finally, the PTCH mutations fit Knudson's two-hit hypothesis that tumors in inherited cancer predisposition syndromes have mutations of the same gene as sporadic ones in the same tumors [29, 38-29]. 3.5 Stem Cell and Hypothesis-related Studies Coller et al. and Sudo et al.'s clustering of mutant mitochondrial DNA copies [40- 41, 5] and stem cells [42], Gostjeva-Thilly's stem cell and multiple human fetal organs [12], and Sudo et al.'s fetal-juvenile origin of nuclear point mutations and lung cancer [5], will support the Gostjeva-Thillly hypothesis that cells with bell-shaped nuclei are 36 responsible for net growth and differentiation in the embryonic gut, adenomas, and adenocarcinomas, and be used to fulfill the requirements for post-embryonic stem cells in carcinogenesis [1-2]. Chapter 5 will devote to the analysis of the Gostjeva-Thillly hypothesis of the fetal-juvenile mutability hypothesis and its inference to ageindependence in skin cancer. 37 Chapter 4 Multiple Genetic Hit Process of Skin Cancer Development 4.1 Multiple Genetic Hit Theory of Cancer Development Carcinogenesis is caused by a multistage process, in which mutations play a pivotal role in cancer development [43]. The stepwise process usually starts with the accumulation of mutations that promote clonal selection of cells and eventually leads to a lethal metastic tumor [3-4, 9, 38, 44-48]. 4.2 Multistage Process of Skin Cancer Development Since sunlight - the ultraviolet radiation (UVR) from the sun-exposure - is the most prevalent human carcinogen, skin cancer is considered an ideal case to study its multistage process leading to skin carcinogenesis. Brash et al. have stated that the stages starting with genetic events, which are preceded by molecular events that create the mutation. Cellular events follow and allow a cell to acquire another mutation to progress to a tumor, as seen in the following Figure 8, while illustrate the genetic and cellular events in the early development of skin cancer [48]: 38 Figure 8: ULTRAVIOLET RADIATION DNA LESION MUTATION (Mutationfrequencies after UVB irradiation: 10~5~ 103/geneper cell generation) GENE CELL PHENOTYPE Clonal Expansion PRECANCER OR CARCINOMA Based on Brash et al.'s "Genetic and cellular events in the early development of skin cancer" [48]. 39 Summarizations of Figure 8 are as follows [48]: 1. Sunlight and its ultraviolet radiation acts as the initiator. 2. Sunlight creates first cyclobutane dimmers and photoproducts in DNA [18], leading to C-T mutations at dipyrimidine sites [49] in the p53 and PTCH tumor suppressor genes [10, 17, 23, 50]. 3. The expansion of a single mutant cell to a clone of 1,000 cells allows one of the 1,000 cells to begin another pass through the mutation loop. 4. One phenotype of losing p53 is the loss of UV-induced apoptosis of DNA-damaged cells resulting in the accumulation of mutant cells [17]. 5. Subsequently, the clonal expansion of the mutant cell leads to the acquisition of a second mutation - which makes the accumulation of multiple mutations possible [48]. 6. Finally, as a promoter, sunlight will continually creates mutant keratinocytes, and drive their clonal expansion [48]. In reviewing the chart above, "clonal expansion" is the key step for the multiple genetic hit mechanism in skin cancer development. In the following, the topic of clonal expansion relating to multiple genetic hit carcinogenesis will be analyzed further. 4.3 Sunlight, Clonal Expansion, and p53 4.3.1 On p53 Gene, p53 Mutations, Frequency, Selection, and Clonal Origin 40 SCC: The p53 is the most studied tumor suppressor gene. It is involved in both BCC and SCC [46]. Brash and Ziegler et al. also observed that over 90% of human SCCs have the p53 mutations induced by ultraviolet radiation [10, 50]. Furthermore, p53 mutations are present in carcinoma in situ [7]. The p53 protein is a transcription factor whose targets include genes that regulate cell cycle and cell death [7]. In addition, over 60% of dysplasias contain p53 mutations [7, 17]. According to Brash et al., the presence of p53 in dysplasias reveals that these precancers are clonal events rather than toxicity reactions. Different parts of the same dysplasia have identical mutations, confirming a clonal origin [7]. However patients with multiple dysplasias have different p53 mutations in each lesion suggesting that each dysplasia originated from a distinct mutational event [17]. Since each mutation changes the amino acid, it appears that the mutations have been selected as opposed to being an incidental side-effect of dysplasia [7]. BCC: p53 mutations are also present in nearly all BCCs, including small, presumably early lesions [26, 51]. In all cases, p53 mutations surface through the lesion suggesting that the mutations arise prior to the emergence of the dysplasia. To further characterize the onset of the mutation and determine whether dysplasia arising within a large p53 mutated clone of cells in skin damaged skin, Ziegler et al examined biopsy samples of normal skin surrounding the dysplasia for the same mutation as that of each dysplasia [17]. The frequency of such mutations are found from ~ 10 4 in Brash et al., and from 10 3 to 102 in Gailani et al. study [8, 10, 36], suggesting that the p53 mutations arise at the same time as the clonal expansion of the tumor. Frequency of mutations is presented 41 10 4 to 10- in Jonason et al study - to be higher in sun-exposed skin - for example, from than in non-sun-exposed skin [37, 8]. Hotspots: Mutations in SCC and BCC are clustered into hotspots with the amino acid substitutions spread evenly across the p53 gene suggesting that some p53 alleles can lead to an SCC, whereas others are more likely to regress [17]. Another possible explanation, coinciding with the findings of Thilly group, is that the regressed p53 mutations occurred in non-stem cells, leading to their ultimate turnover and disappearance from the skin tumor [5]. 4.4. Sunlight, Clonal Expansion, Apoptosis, Stem Cell Compartment of the Epidermis The acouisition of a second mutation: Studies about the sun-exposed skin of Caucasians contain thousands of p53-mutated clones, which are clinically invisible but can be recognized in skin immunostained with the p53 antibody. UV-related p53 mutations -C to T or CC to TT transitions at dipyrimidine sites -- are confirmed in the p53 mutated clones by microdissection and sequencing [7-8, 10, 17, 33]. The p53 gene is critically involved in the regulation of cell cycle control and apoptosis. Mutations in the p53 gene could potentially abrogate p53 function, allowing for the accumulation of mutant cells due to loss of the UV-induced apoptosis of DNA-damaged cells. This leads to clonal expansion of p53-mutated keratinocytes, creating a larger target for the acquisition of a second mutation. The second mutation will lead to the progression of clinically recognizable solar keratosis. 42 4.4.1 UV Drives Clonal Expansion of p53-mutant Keratinocytes in Normal Skin The results reported by Brash, Ponten, Ren, Jonason, and Ziegler et al. have shown that epidermal p53 clones are frequently detected in chronically sun-exposed sites [7-8, 2224, 50, and 52]. Furthermore, the data presented by Brash et al. have shown that heavily sun-exposed skin would be more likely to sustain a mutation early in life, and that these early cells could grow into a clone even without further sunlight [48]. The study conducted by Jonason et al. revealed a much comprehensive report of the multiple genetic hit model of carcinogenesis which predicts that ordinary people have cancer-prone mutant cells dormant for further genetic hits [8], consistent with Marks, Kricker, English or Ziegler et al. claim that critical sunlight exposure is received before the age of 18. In addition, Jonason et al.'s article furnished an invaluable link between the time of initial sun-exposure and subsequent skin cancer years later, claiming that sunlight acts as both a tumor initiator and tumor promoter by favoring the clonal expansion of p53mutated cells. In their study, both patch size (sun-exposure dependent) and frequency are independent of age [8]. 4.4.2 Clonal Expansion involving UV-induced Physiology, not Mutation In their study of the colonization of adjacent stem cell compartments by mutant keratinocytes, Brash, Jonason et al. provided detailed counts of clone sizes that indicate the quantized distribution of sizes of p53-mutant clones. They stated that the occurrence of quantized clone sizes reveals the fact that a rate-limiting step in clonal expansion is the ability to expand beyond the first stem cell compartment [48]. 43 UVB exposure drives clonal expansion by a non-mutational mechanism, allowing mutant stem cells to escape from their own stem cell compartment and colonize adjacent compartments - similar to J. Cairns' safety mechanism proposed in 1975 [53]. 4.4.3 Remarks on Clonal Expansion and UV-induced Apoptosis The regular role for UVB-induced apoptosis is to delete DNA-damaged cells in unmutated stem cell compartments, while sparing death-resistant p53-mutant cells [17, 48]. Consequently, the effect of apoptosis on cancer development is stage-specific: apoptosis suppresses two stages of cancer development that require new mutations (i.e initiation and malignant conversion). However, apoptosis can drive clonal expansion, for both existing p53-mutant cells and for papilloma precursors [48]. Brash et al. pointed out that the additional source of apoptotic selection pressure may come from UV-irradiated melanin [48]. The conclusive remarks for this chapter are that ultraviolet radiation is the carcinogen for human skin cancer, and clonal expansion is required for the multiple genetic hit mechanisms for cancer development. As an initiator, this UVB creates a mutant cell, which can drive clonal expansion of the single mutant cell later on. The other function for sunlight, including both UVB photosensitization reactions. and UVA, is to induce apoptosis by melanin This will create the opportunities to investigate new non- mutational pathways and to accelerate the clonal expansion step of multiple genetic hit carcinogenesis [48]. 44 Chapter 5 Application and Calculations 5.1 Justification and Application Approach Since the mid-1980s, a stream of researchers -- such as Marks, Kricker, Kreamer, Ziegler, Laffell, English, Jonason, Nakazawa, Ouhtit, Ren, Yamasaki, Ponten and Brash, among others have reported that the distinctive mutations initiated by UV light allowed early events inferred from mutations observed in tumors [8, 10-12, 15-17, 20-21, 36, 43, 54-59, and 61-68]. In other words, they furnished new insight into the link between sun exposure and skin cancer, and analyzed events occurring between the initial sun exposure received before the age of 18 by normal individuals and subsequent skin cancer occurrence years later [8, 12, 20, and 73]. For instance, research by Jonason et al. has shown that since keratinocytes are continuously lost through squamous differentiation, it is likely that the cell which is targeted by sunlight decades before the tumor's appearance is a stem cell [8]. Accordingly, the study suggests that the keratinocytes contain the mutations measured in normal skin reside in clonal patches originated from mutated stem cells [8]. This coincides with Gostjeva-Thilly's study of bell-shaped nuclei and the fetal-juvenile mutator stem cell hypothesis, which states that both net stem cell growth and discernible point mutation 45 appear to have ceased at the juvenile-adult transition (around 15 to 18 years of age), and are not affected by the age, gender, or smoking or non-smoking status [1-2, 5]. Accordingly, three approaches will be taken for this chapter: (1) In order to apply Gostjeva-Thilly's age independent hypothesis to this current study on skin cancer, this chapter will first revisit and reanalyze research by Brash, Poten, Zielgler et al. on the influence of age on frequency and size of epidermal p53 clones via Jonason et al.'s study on "Frequent clones of p53-mutated keratinocytes in normal human skin" [8]. (2) In order to examine Jonason et al.'s presented data, I will re-calculate their data related to the patch frequency with respect to age, as well as with respect to the three categories of sun-exposed data: sun-shielded, intermittently sun-exposed and chronically sun-exposed in a graphed format to compare with Jonason et al.'s findings [8]. (3) In the "Discussion" section, this study will infer to Gostjeva-Thilly's fetal-juvenile mutator stem cell hypothesis. By utilizing Jonason et al.'s data, my calculation presented in graph format will confirm that both Jonason et al.'s explanation on the age independence of clone frequency, and Gostjeva-Thilly's hypothesis indicating that mutations rarely occur in adult maintenance stem cells that occur at significant constant rates in pre-adult stem cells involved in fetal and juvenile growth [1-2, 5]. 46 5.2 Example On "Frequent Clones of p53-mutated Keratinocytes in Normal Human Skin" by Jonason et al [8]: This portion of 5.2 is based on Jonason et al's findings. 5.2.1 Materials and Methods: 1. Jonason et al. devised a whole-mount preparation method for human epidermi collected from volunteers, none of whom had skin cancer. They used this method to assist immunohistochemical analysis for stabilized p53 protein. Usually a p53 mutation could lead to nuclear immunopositivity. 2. DNA amplification and sequencing: DAB-stained patches were microdissected with 30 gauge steel needles, while the total genomic DNA was amplified to a copy number sufficient for multiple p53specific PCRs. Furthermore, reamplification for exons of the p53 gene was performed with primers, amplification condition, and controls for contamination and polymorphisms, as were direct DNA sequencing of uncloned PCR products and sequence confirmation. 3. Statistical analysis: In this area, statistical analyses were performed with patients as the units of analysis. a. Using Wilcoxon rank sum test for comparisons of patch frequency and patch size between 2 levels of sum-exposure. b. Using the Jonckheere-Terpstra test to perform the test of dose-response. c. All "P" values were two-sided. 5.3. p53-Mutant Clones, Sun Exposure, Clone Number, Size, and Frequency 5.3.1 p53-Mutant Clones The results of the experiment by Jonason et al. are as follows: Many p53-immunopositive patches are visible by light microscopy. These patches are seen in both the dermal-epidermal junction and the hair follicle, as shown in Figure 9: 47 Figure 9: .JA A AV 40. p53-immunopositive clones in whole-mount preparationsof human epidermis. Clones arising from (A): the dermal-epidermal junction, and (B): a hair follicle. In both cases, view is from the basal surface to show follicles. (x100.) From PNAS 93: 14026 (1996) by Jonason et al. [8] The dose-response of patch frequency is statistically significant, P = 0.0001. difference between each pair of sun-exposure categories is P = 0001 to 0.01. The Three categories of clone frequency: Sun-shielded, Intermittently Sun-Exposed and Chronically Sun-Exposed - ranged from an average of 3 patches per cm 2 in sun-shielded skin to 33 patches per cm 2 in chronologically sun-exposed area, as shown in Figure 10: 48 Figure 10: Increase of clone frequency with sun exposure 40 K l 30 E CL 10-- 0 Sun-shielded Intermittently sun-exposed Chronically sun-exposed - 53 Increase of clone frequency with sun exposure. Histogram shows the average frequency of p mutated patchesfrom sun-shielded, intermittently exposed, and chronicallysun-exposed skin. Mean patchfrequency for intermittently excludeds the outlier. Bars represent SEM. From PNAS 93: 14026 (1996) by Jonason et al. [8] Furthermore, it presents that the patch frequency is independent of age. Two distinct morphological patterns are presented: diffused and compact. In order to determine whether the patches are in fact composed of p53-mutated cells, Jonason et al. microdissected 16 stained compact patches from sun-exposed skin, randomly amplified the entire genome, and then amplified specific exons of the p53 gene. Following direct DNA sequencing of uncloned PCR products, they report that 50% of the patches contain a mutation in the p53 structural gene as shown in Figure 11 below: 49 Figure 11: Patches of p53-mutated keratinocytes in human epidermis by Jonason et al: N" A '-ol ok.;1, it ;. t, I;I. idI IJ~i ILL' N I I _ .hill - 1 I N' N I, ::44.1 T' 'Ik - *.'i TT G(I N.A I v t S.I 'sIn S11|1! IGiu (iIy 2'' -(M i mli ) 'L f I Ni tN (~ - - . pp Ii I A:.' Ar'4 L L i 2 :.' ",' I '1 NI I NI .4 *III''.1A : V'1 7 ICh 4 C %' - t. * I**'wI I.,p e ceid k . h '.1I -1.41 iieli i ! .4. 'IL IC '41I i I. 114.7 0. I N1- -. .7 . i4 I : IV I' 4 . k-AkI p1 : iik i ne '.. .C. 4.4 4".,]I~ l :CI l SII ? 4f~ 10.1 c'e k. lil -. +ncc 2 . N C ? d4.I4t.W14.4'.U . 111d 1 r e N il. .h -- p. 11k %. .. 117i 0, 1111l From "Frequentclones ofp53-mutated keratinocytes in normal human skin" by Alan S. Jonason, et al. PNAS, USA 93: 14027 (1996) [8] 50 il-- 11I 1 11.111r: They also reported that nonstaining skin flanking the patches is wild-type, indicating that each p53 mutation is limited to a patch. The intensities of the mutant and wild-type DNA sequencing bands are equal, indicating the entire patch is occupied by heterozygous mutations, and each patch is a clone. In comparison, these results are similar to the report from Ren et al. in "Two distinct p53 immunohistochemical patterns in human squamous-cell skin cancer, precursors and normal epidermis" [52]: 1. The dispersed pattern shows no correlation to the age of the individual; 2. The presence of p53 immunoreactivity as a compact pattern supports the theory that mutations of the p53 gene are early events in the sequence from dysplasia to invasive squamous-cell cancer of the skin. In addition to Ren et al.'s, various publications cited in the beginning of this chapter echo a similar assessment of the influence of age on frequency and size of epidermal p53 clones. 5.3.2 Number and Size of Cells in the Clones _Jonason et al. stated that in order to estimate the number of cells in these clones, they viewed them from the basal surface and counted 6,400 mutated cells per mm2 These mutant colonies have a size from 60 to 3000 cells, exceeding frequencies of 40 cells per cm 2 involving as much as 4% of sun exposed, normal epidermis. 51 The size of the clone is larger in chronically sun-exposed skin than in intermittently-exposed skin, suggesting that sunlight not only initiates but also drives clonal expansion. See following Figure 12: Figure 12: Increase of clone size with sun exposure .25 EN 0.2 E E 499 Sun-shielded Intermittently sun-exposed Chronically sun-exposed Increase of clone size with sun exposure. Dot histogram shows the area in mm2 of individual p53-mutatedpatchesfrom sun-shielded, intermittently exposed, and chronically sun-exposed skin. Each point represents an individualclone. From PNAS, USA 93: 14028 (1996) [8] Furthermore, these cells are arranged spatially as conical clones arising from stem-cell compartments and supporting the idea that stem cells are the cell of origin [8]. These p53mutant stem cells, while Marks and Kricker note, offer a clear explanation of the ability of childhood sun exposure to affect adult cancer frequency [12, 20]. 52 EPU size and stem cell compartment. In the absence of UV, their growth is halted and the clones regress. Interestingly, the initial size of the p53-mutated clone is similar to the size of an EPU, or epidermal proliferating unit (i.e. one stem cell compartment), indicating that the stem cell compartment of the epidermis acts as a physiological barrier to clonal expansion of p53-mutated keratinocytes as reported by Zhang et al. [74]. Sustained UV exposure deletes neighboring stem cell compartments by apoptosis, thereby enabling p53-mutated keratinocytes to colonize an adjacent compartment without incurring an additional mutation [74]. Average patch size for each level of sun exposure was taken from the data of Jonason et al. According to the Gostjeva and Thilly's fetal-juvenile theory, the sun shielded patches would best represent the preneoplatic lesions that occurred before maturity. The intermediate and chronic patches represent the preneoplastic lesions after having been exposed to a mutagen - the sun. From Figure 13, it can be seen that the average size of the sun shielded patch is much smaller than the size of the intermediate or chronic patches, which are about equivalent in size. This finding is consistent with the fact that the sun shielded patches were excised from locations in which the preneoplastic lesion would not have been exposed to growth inducing rays from the sun, whereas the intermediate and chronic patches may have started out with similar sizes as the sun shielded patches, but after prolonged exposure to the sun, consistent with the fact that sunlight can be categorized as a carcinogenic promoter. 53 Figure 13: Average Patch Size According to Sun Exposure 0.045 0.04 < 0.035E 0.03 Intermediate, 0.0407 Chronic 0.0402 + 0.025 Amount ofSun Exposure Sun Shielded, 0.0208 0.02-Fn 0.015 -8 0.01 am 0.0050 _ Amount of Sun Exposure To test this hypothesis further, the log2 of sun shielded cluster size in mm was graphed verses the log2 of cluster number based upon the same data from Jonason et al. Figure 14 shows a linear fit of these values (y = 0.0188x - 0.1149) with a slope close to zero, indicating the absence of change of cluster number based on cluster size, consistent with the fact that these sun shielded patches were rarely exposed to the sun's carcinogenic rays. Figure 14: Sun-Shielded Log(2) Cluster Number/Size 0.16 . 0.14 E 0.12 ) U) E 0.1 0.08 - Z - 0.06 - 0 0.04 Vj 0.02 Sun-Shielded - Linear (Sun-Shielded) y= 0.0188x - 0.1149 R2 = 0.7985 0 -j -0.02 - 0 2 4 6 8 Log(2) Cluster Size 10 -12 Simlarly, Figures 15 and 16 show a similar graph of the log2 of cluster size versus the 10g2 of cluster number. However, the cluster numbers for the intermittently and chronically exposed patches were divided by the average cluster size for the sun shielded patches. This calculation corrects for the growth of the patches due to sun exposure so that cluster number and size can be compared. The lines of best fit were found for the intermittently shielded (y = 0.9874x - 8.0092) and chronic (0.999x - 8.0566) skin patches. The slopes for these lines are almost linear, suggesting that cluster size increased with amount of sun exposure. Figure 15: Chronic Sun Exposed Log(2) Cluster Number/Size 4 3Z 2 Chronic Sun Exposed 0 Linear (Chronic Sun ------ S2 0 .. 4 6 10 -2 1Exposed) y = 0.999x - 8.0566 -3 R2 = 0.9739 Log(2) Cluster Size 55 Figure 16: Intermittently Shielded Log(2) Cluster Number/Size 4 3 n E 2 z -- +-- 1 Linear (Intermittently 0 -1 Intermittently Shielded 4 6 10 ' 2 0 -2 -j 12 Shielded) y = 0.9874x - 8.0092 -3 R2 = 0.9783 Log(2) Cluster Size 5.4. Patch Frequency is Independent of A2e The age independence of clone frequency presented by Jonason et al. is very much in accordance with Kricker's report on substantial precancerous effects during childhood [20], in consistence with Marks' human skin precancers report [21], and with Berg's findings of early p53 alterations in mouse skin carcinogenesis by UVB radiation by immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells [59]. In measuring p53 patch frequency, consideration must be given to the total area of p53-immunopositive clones in a skin sample which constitutes a measure of p53 mutation 56 frequency. In other words, the patch frequency is calculated by averaging the clone areas and then multiplying by the number of clones per cm 2 in the same skin sample. As reported that the patch size often varied - 20-fold in the same person. The area of each measured patch is plotted in the histogram, as seen in the previous Figure 12. Clearly, each sun-exposure category contains a different distribution of patch sizes. Since sunlight plays the pivotal role as the mutagen, the average patch size is larger in chronically sun-exposed skin than in sun-shielded skin (P= 0.02). Specifically, Jonason et al.'s calculated that the frequency of patches exceeding 0.05 mm 2 is 5 times greater in chronically exposed skin (25%) than in sun-shielded skin (5%) (P= 0.04 by Fisher's test), with intermittently sun-exposed skin falling in the middle. The combination of both size and frequency has proved that sunlight also plays a promoter role in the promotion of the clonal expansion of p53-mutant cells [8]. Statistically, the p53 mutation frequency ranged from 10- to 4 x 10-2 in sunexposed skin and 10-4 to 10- in sun-shielded skin. It is estimated that around 4% of sunexposed normal epidermis contains p53-mutated keratinocytes [8]. In measuring each category, the frequency of patches ranged from an average of 3 patches per cm 2 in sunshielded skin to 33 patches per cm 2 in chronologically sun-exposed area. The following Figure 18 is calculated to verify and prove that Jonason et al.'s patch frequency is independent of age report. Data are based on Figures 9, 10, 11, 12. The solid line indicates line regression computed for three aforementioned sun-exposure categories: Sun-shielded, Intermittent, and chronically sun-exposed skin. The blue- colored diamond shape is for sun-shielded, red square for intermittent, and yellow triangle 57 for chronically-exposed. The results are consistent with Jonason et al. data presentation for mutant frequency and age independence theory: Figure 18: Age Independence and Patch Frequency - - - - + Sun-shielded M Intermittent - 50 45 40 35 0 30 25 LL +20 15 a. 10 5 Chronic . -Linear - Linear (Intermittent) - - Linear (Chronic) - 0 (Sun-shielded) - 0 20 40 60 80 100 Age It can be seen that the lines of best fit for the sun-shielded (y = 0.1047 - 0.5624, R2 + 0.1244), intermittent (y = 0.0646x + 10.674, R2 = 0.2811) and chronic (y = -0.1227x 39.649, R2 = 0.0579) sample groups have slopes close to zero, indicating the absence of change in the number of lesions as age of the patient increases. This data corresponds with the fetal-juvenile mutability hypothesis and the conclusions made by Sudo et al. for constant cluster number in the lung irrespective of age. 58 5.5. Inference to the Fetal-Juvenile Mutability Hypothesis and Discussio The observations presented by Jonason et al. have provided an analogous to lung cancer and colon cancer studies conducted in the Thilly lab. Jonason et al.'s age independent statistics in reference to normal tumor skin, clonical patches, size and frequency are comparative to the research findings in lung epithelium studies, or colon cancer studies [9, 5, 1]. For example, the age independence of p53 clone frequency and the occurrence of substantial mutagenesis arising during childhood indicating that mutagenicity are proportional to the net cellular growth rate [9]. This growth rate of normal juvenile (0.16 doubling/per year) is very similar to the growth of peneoplastic lesion in smokers (0.17 doubling/year) [9]. In addition, measurements taken from the lung has led Sudo et al. to infer that smoking and aging have no effect on the measured point mutant fractions nor on their numerical distributions in the adult lung epithelium, leading to the conclusion that the point mutations must have occurred before maturity. From this data, it is further suggested that the mutant fractions are constant with age in adults because mutations occur much less frequently in adult maintenance stem cells than in pre-adult stem cells involved in fetal and juvenile growth. Furthermore, Sudo et al. proposed that mutations arose during an exponential increase in stem cell number during human organ growth and development [5]. This has been further observed by Gostjeva-Thilly in their stem cell stages and the origins of colon cancer study [1']. Their calculation confirms that the calculated rate of growth of preneoplastia is equal to the calculated growth rate of the juvenile colon, indicating that the tumor initiation actually blocks the development process by which 59 growing juvenile stem cells are transformed into or replaced by adult maintenance stem cells [1]. In conjunction, Gostjeva-Thilly group's joint publication reports the discovery of the multiple forms of clear and reproducible nonsperical nuclei present in the fetal gut (see Figure 19 below), colonic adenomas and adenocarcinomas that appear to be absent in normal adult colonic crypts [2]. Named "metakaryotic", these amitotic cells with stem-like qualities have been observed in high frequency in fetal, preneoplastic and neoplastic tissues of the colon, but not in the adult colon. With non-spherical, bell-shaped nuclei and peculiar "cup-from-cup" forms of symmetrical amitotic fission, these cells also display a form of amitotic asymmetrical nuclear fusion in which multiple nuclear morphotypes populate a fetal organ by emerging from the bell-shaped nuclei [5]. Based on these findings and comparisons, it can be suggested that skin cancer in adults receives its first mutations in the metakaryotic stem cells of the fetal-juvenile state and is able to maintain the positive cellular growth rate of juvenile, capable of developing to preneoplasias [9]. This finding would validate the epidemiological observations, particularly seen in white Australians, in which children but not adults exposed to nuclear radiation or excessive sunlight have greater numbers of tumors per person than their genetic peers that where not exposed to excessive exogenous agents during the fetaljuvenile period. 60 Figure 19: Nuclear fission of bell-shaped nuclei in the fetal gut Ilk 4, 0 Nuclearfission of bell-shaped nuclei in the fetal gut: (a and b) Symmetrical nuclearfission: bell-shaped nuclei emergefrom bell-shapednuclei of similarshape. (c and d) Assymetrical nuclearfission: a spherical nucleus and cigar-shapednuclei emergingfrom a bellshaped nucleus. Scale bar, 5 pm. From "Bell-shaped nuclei dividing by symmetrical and asymmetrical nuclear fission have qualities of stem cells in human colonic embryogenesis and caracinogenesis" by E.V. Gostjeva, L. Zukerberg, D. Chung, and W.G. Thilly. Cancer Genetics and Cytogenetics 164: 16-24 (2006), p. 20 and 21 [2]. They further stated that such structures might bear on the hypothesis that tumors are a re-expression of embryonic phenotypes, specifically tissue stem cells forming clonal population with derived differentiated cellular phenotypes [2]. These studies published 61 between 2005 and 2008 [1-2, 5], help to resolve the assumptions and attributions related to skin cancer's cell of origin, stem cell of fetal organogenesis, adult maintenance stem cells, stems of juvenile growth and stem cells of fetal organogenesis relating to sunlight initiation, promotion, mutant cells, clonal size, frequency and age independence and expansion - questions raised by skin cancer researchers from Brash, Ponten, Zigler, Jonason to Marks, or Krickers, et al. In fact, Gostjeva-Thilly's study of metakaryotes, bell-shaped nuclei and the fetaljuvenile mutator stem cell hypothesis indicates that cells with bell-shaped nuclei are responsible for net growth and differentiation in the embryonic gut, adenomas, and adenocarcinomas, and fulfill the requirements for post-embryonic stem cells in carcinogenesis [1-2]. In the following table and diagram below, Figure 20 and 21 summarize stem cells and their qualities in colon fetal development and carcinogenesis observed in histopathological specimens, and a pediatric growth charts revealing average juvenile growth to be a simple exponential function of age between 18 months and maturity [1]: 62 Figure 20: Bell-shaped nuclei with higher frequency in "undifferentiated" areas within adenocarcinomas: Tilbl: I I Th .i I ' I QLIJ It k' 11 I . Ie 1 F.'t Vi.Iv PIn11 .I)1t and (.,1 ci0f(enC 1 Ubscr~cd in Hist I.thai giCal cw .,:N i: r~epas 1. PIfK. Ltitld I., I'I.t. t 4 cci- a I AtdL AduIt I t :: 1,t JLt CL' et;dD~ ||1Jte4j'pf 111C. I- It I s cc -.ta i b l CV FIIr hN 2 I- cryp:tt,,+lILI 0 A-y *1110tee 2 .( v~~~~~~ i~ ' itV :0.m. 'Ne. NaiL' IIICIL etaIl Id idi: r h tufmor ... xt Ir ti d d,1b ing I/i tl~ 11 11 II bl I . U1 lI 1 11 iI GostjevaandWilliamG.Thilly Stem ellI Rviws 0 Vol.- U1, c harts Figure 211 :'%ILr Peit IL goth I'-,.t.~i:i til cel Is(4 li VLX ci tLILIi1 -1101'liN tetl ~~ ~ "'.cI:''3I~aIIi:I'r SpecImens ai I 1Ci'H \01k I ; p. I2605 ] Sio'l t ili iLIluItI dit d ilu tf ~ Ama't F III" 0,VVlU le ICy tJilglliielt I.1 l i 'll % to Fromltem dCe0ll Stage anthe Or-igins of olon Canler: \uilee "Fig It. d Ithirv in i todf l iltyke _i 1-1 AlI(4nflielit- '114121 , withi Ahlgnlnlent 1 4 fe jr- ill lenae V. NA Mulisciln Perectie"y v GH Wtth"ledtOt (Ig Jh',4111I)C-1t Oi 11LClIA o I.,' evi denlt From "Stem Cell Stages and the Origins of Colon Cancer: A Multidisciplinary Perspective" by Elena V. Gostjeva and William G. Thilly. Stem Cell Reviews, Vol. 1, p. 246, 2005 [1]. Figure 21: Pediatric growth charts: A ii. From "Stem Cell Stages and the Origins of Colon Cancer: A Multidisciplinary Perspective" by Elena V. Gostjeva and William G. Thilly. Stem Cell Reviews, Vol. 1, p. 246 , 2005 [1]. - B Fig 2 Pedlarr i growth r.e US ci ,verage 'er'een I 995 uveile 111'.) o mile (A) ind feirnle (B) hots plorred l oeii coordijtes rowth t JId ro uNtuIcy b.? IinplI expienirmal children of These data reveal funcion of aqg'I 63 1' - 1o Specifically, several key points related to skin cancer can be inferred to the Gostjeva-Thilly hypothesis [1]: 1. Tumor initiation and promotion issue: can be explained by Gostjeva-Thilly's discussion on growth, differentiation in fetal, neonatal, and juvenile development. 2. Mutation of preadult stem cells: are embodied in these bell-shaped nuclei as observed in fetal tissue and tumor samples. 3. Initiated "juvenile" stem cells: can be explained by the continuation to create local patches of juvenile tissue, later on observed as polyps 4. Mutator phenotype in normal juvenile tissue, observed in these bell-shaped nuclei express: can be explained as maintained in preneoplastic lesions. 5. The slow growth of the preneoplastic colony: can be explained by at least one of the preneoplastic juvenile stem cells to a fetal stem cell phenotype that rapidly creates a lethal tumor mass. 6. Testing the hypothesis tool for tumor initiation occurring at higher rates in juvenile stem cells than in adult maintenance stems: can utilize MAMA, PCR, CDCE technologies originated from the Thilly Lab. Accordingly, two key questions raised in this study are responded by the inference to Gostjeva-Thilly's fetal-juvenile mutator stem cell hypothesis: 1. The keratinocytes containing the mutations measured in normal skin reside in clonal patches originated from mutated stem cells: Gostjeva-Thilly explain that the stem-cell mutation limited to the fetaljuvenile period. In addition, their report also indicates that a stem-like cell form in multiple human fetal organs suggesting that point mutagenesis of the epithelia of both the skin and lung are restricted to the stem cells of the fetal-juvenile period [5, 1-2]. 2. Age independence issue: Gostjeva-Thilly explain that point mutant fractions are constant with age because mutations rarely occur in adult maintenance stem cells but frequently occur at constant rates in the transition cells of the turnover unit. Consequently, the age-invariant total nuclear mutant fractions and their numerical distributions as clusters are not accounted by a steady state process of mutations in transition cells of adult lung epithelial turnover units. [5]. In addition, The average rate of p53 gene inactivating point mutation per stem cell gene copy is calculated to be -1.6 x 104 per stem cell doubling. Both net growth 64 and discernible point mutation appear to have ceased at the juvenile-adult transition and are not effected by aging, gender [5, 1-2]. 65 Chapter 6 Remarks and Conclusion Cancer is a disease caused by multiple genetic hits in which mutations play a pivotal role. These multiple genetic hits models study the evolutionary process of tissue cells in an individual's organ from a normal stage to an initiated pre-neoplastic stage, and finally promote to a neoplastic stage that results in tumorgenesis [4]. Furthermore, t'he implication of these models can predict that normal individuals shall have stable populations of cancerprone, but noncancerous, mutant cells awaiting for further genetic hits [8]. In the case of skin cancer development, epidemiological and molecular data clearly indicate that sunlight is a carcinogen and the primary cause of skin cancer in which forms of point mutation associated with ultraviolet radiation, and acts as both a tumor initiator and a tumor promoter by favoring the clonal expansion of p53 mutated cells [10, 17]. Addressing these premises in terms of the distribution of mutant cells in human skin along with the exploration of the fetal-juvenile mutability hypothesis, this study has been approached from three angles. First. In order to support the idea that sunlight's dual roles provide a selective growth advantage to both endogenous and exogenous mutant, this study utilizes Jonason et al.'s report [8], which indicates that normal human skin contains multiple colonies of keratinocytes that stain for aberrant p53 protein. These mutant colonies are larger in size and higher in frequency in sun-exposed skin than in sun-shielded 66 skin, suggesting that sunlight both induces p53 mutations and stimulates the expansion of mutant colonies. These mutant colonies have an average size of 60 to 3000 cells, exceeding frequencies of 40 cells per cm 2 involving as much as 4% of sun exposed and normal epidermis. In addition, since the patch size often varied 20-fold in the same individual, Jonason et al's observation is in accordance with Thilly group's claim regarding large variances of mutant cluster sizes (in the normal lung epithelium) [5, 8]. Second: The age independence of clone frequency presented by Jonason et al. is very much in accordance with Kricker's report on substantial mutagenesis during childhood [20], and consistent with Marks' human skin precancers report [21] and Berg's findings of early p53 alteration in mouse skin carcinogenesis by UVB radiation in clusters of preneoplastic epidermal cells [59]. Other quantitative detections of p53 gene mutation in normal skin studies conducted by Nakazawa, Ouhtit, Yamasaki et al. [54-58], or mitochondrial DNA deletions in human skin by Birch-Machin [75], all indicate that no statistically significant association could be demonstrated between p53 mutation frequency and age, gender, sun sensitivity, or total exposure to the biopsy site. In view of the extremely high mutation frequency in his study, Nakazawa et al. suggest that selective clonal expansion of mutant cells may have occurred in vivo [57]. In short, Jonason et al.'s report on the high frequency of aberrant p53 clones in human skin is independent of age among adult. My data analysis in has also verified his theory. Third: The distribution of mutant cells in human skin, along with the first and second remarks numerated above, are justified by Gostjeva-Thilly's fetal-juvenile mutability hypothesis. 67 Study conducted by Jonason et al. further indicates that (i) the cell which is targeted by sunlight decades before the tumor's appearance is a stem cell; and that (ii) the keratinocytes contain the mutations measured in normal skin reside in clonal patches originated from mutated stem cells [8]. These two items can be verified by the GostjevaThilly's study of bell-shaped nuclei and the fetal-juvenile mutator stem cell hypothesis, which states that both net growth and discernible point mutation appear to have ceased at the juvenile-adult transition and are not affected by the age, gender, or smoking or nonsmoking status [5, 1-2], In conclusion, both the observation of Jonason et al. in nuclear mutation in the skin and Gostjeva's report of a stem-like cell form in multiple human fetal organs suggest that point mutagenesis of the epithelia of both the skin and lung are restricted to the stem cells of the fetal-juvenile period. Finally, the impact of Gostjeva-Thilly's report on the discovery of amitotic "metakaryotic" cells with stem-like qualities in fetal and preneoplastic tissues of the colon cannot be underestimated in cancer research.. 68 References 1. Gostjeva, E.V. and Thilly, W.G. Stem cell stages and the origins of colon cancer: A multidisciplinary perspective. Stem Cell Reviews 1: 243-252 (2005). 2. Gostjeva, E.V., Zukerberg, L., Chung, D., and Thilly, W.G. Bell-shaped nuclei dividing by symmetrical and asymmetrical nuclear fission have qualities of stem cells in human colonic embryogenesis and carcinogenesis. Cancer Genetics and Cytogenetics 164: 16-24 (2006). 3. Herrero-Jimenez, P., Thilly, G., Sosutham, P.J., Tomita-Mitchell, A., Morgenthaler, S., Furth, E.E., and Thilly, W.G. Mutation, cell kinetics, and subpopulations at risk for colon cancer in the United States. Mutation Research 400 (1-2): 553-578 (1998) 4. Herrero-Jimenez, P., Tomita-Mitchell, A., Furth, E.E., Morgenthaler, S., and Thilly, W.G. Population risk and physiological rate parameters for colon cancer. The union of an explicit model for carcinogenesis with the public health records of the United States. Mutation Research 447 (1): 73-116 (2000). 5. Sudo, H., Li-Sucholeiki, X., Marcelino, L.A., Gruhl, A.N., Herreto-Jimenez, P., Zarbi, H., Willey, J.C., Furth, E, E., Morgenthaler, S., Coller, H.A., Ekstrom, P.O., Gostjeva, E.V., and Thilly, W.G. Fetal-juvenile origins of point mutations in the adult human tracheal-bronchial epithelium: Absence of detectable effects of age, gender or smoking status (2008). 6. Thilly, W.G. Have environmental mutagens caused oncomutations in people? Nature Genetics 34: 255-259 (2003). 7. Brash, D.E. and Ponten, J. Skin Precancer. CancerSurveys 32: 69-113 (1998). 8. Jonason, A.S., Kunala, S., Price, G.J., Restifo, R. J., Spinelli, H.M., Persing, J.A., Leffell, D.J., Tarone, R.E., and Brash, D.E. Frequent clones ofp53-mutated Keratinocytes in normal human skin. Proc. NationalAcademy of Sciences 93: 1402514029 (1996). 9. Sudo, H. Point Mutations in normal lungs of smokers and non-smokers. Ph.D. Dissertation, Massachusetts Institute of Technology. Cambridge, MA (2004). 10. Brash, D.E., Rudolph, J.A., Simon, J.A., Lin, A., McKenna, G.J., Baden, H.P., Halperin, A.J., and Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. NationalAcademy of Sciences USA 88 (22): 10124-10128 (1991). 69 11. Marks, R., Jolley, D., Dorevitch, A.P. and Selwood, T. The incidence of nonmelanocytic skin cancers in an Australia population: results of a five-year prospective study. Medical JournalofAustralia 150: 475-478 (1989). 12. Marks, R., Jolley, D., Lectsas, S., Foley, P. The role of childhood exposure to sunlight in the development of solar keratoses and non-melanocytic skin cancer. Medical JournalofAustralia 152: 62-66 (1990). 13. Brash, D.E., Ziegler, A., Jonason, A.S., Simon, J.A., Kunala, S., and Leffell, D.J. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. Journalof Investigative DermatologySmposium Proceedings 1: 136-142 (1996). 14. Brash, Douglas E. Sunlight and the onset of skin cancer. TIG 13 (10): 410-414 (1997). 15. English, D.R., Armstrong, B.K., Kricker, A., and Fleming, C. Sunlight and cancer. Cancer Causes and Control 8: 271-283 (1997). 16. Leffell, D.J.and Brash, D.E. Sunlight and skin cancer. Sci Am. 275 (1): 52-53, 56-59 (1996). 17. Ziegler, A., Jonason, A.S., Leffell, D.J., Kimmelman, J., Remington, L., Jacks T., and Brach, D.E. Sunburn and p53 in the onset of skin cancer. Nature 372 (22/29); 773-776 (1994). 18. Brash, D.E. UV mutagenic photoproductsd in Escherichia coli and human cells: A modecular genetics perspective on human skin cancer. Photochem Photobio48: 5866 (1988). 19. Backvall, H., Wolf, 0., Hermelin, H., Weitzberg, E., and Ponten, F. The Density of epidermal p53 clones is higher adjacent to squamous cell carcinoma in comparison with basal cell carcinoma. British JournalofDermatology 150: 259-266 (2004). 20. Kricker, A., Armstrong, B.K., English, D.R., and Heenan, P.J. Pigmentary and cutaneous risk factors for non-melanocytic skin cancer - a case-control study. InternationalJournalof Cancer48: 650-662 (1991). 21. Marks, R. Premalignant disease of the epidermis. J. Roy. Coll. Phys. (London), 20: 116-121 (1986). 22. Ponten, F., Berit B., Ren, Z., Nist6r, M., and Ponten, J. Ultraviolet light induces expression of p53 and p21 in human skin: effect of sunscreen and constitutive p21 expression in skin appendages. JournalofInvestigative Dermatology 105 (3): 40270 406 (1995). 23. Ren, Z., Hedrum, A., Ponten, F., Nister, M., Ahmadian, A., Lundeberg, J., Uhlen, M., and Ponten, J. Human epidermal cancer and accompanying precursors have identical p53 mutations different from p53 mutations in adjacent areas of clonally expanded non-neoplastic keratinocytes. Oncogene 12: 765-773 (1996b). 24. Ren, Z., Ahmadian, A., Ponten, F., Nister, M., Berg, C., Lundeberg, J., Uhlen, M., and Ponten, J. Benign clonal keratinocyte patches with p53 mutations show no genetic link to synchronous squamous cell precancer or cancer in human skin. American JournalofPathology 150 (5): 1791-1803 (1997). 25. Lajtha, L.G. Stem cell concepts. Differentiation. 14: 23-34 (1979). 26. Jensen, U.B., Lowell, S., and Watt, F.M. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on wholemount labeling and lineage analysis. Development 126: 2409-2418 (1999). 27. Potten, C.S. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Intl. Rev. Cytol. 69: 271-318 (1981). 28. Asplund, A., Gustfsson, A.C., Wikonkal, N.M., Sela, A., Leffell, D.J., Kidd, Lundeberg, J., Brash, D.E., and Ponten, F. PTCH codon 1315 polymorphism and risk for nonmelanoma skin cancer. British JournalofDermatology 152 (5): 868-873 (2005). 29. Gao Ling p53 alterations in human skin: A molecular study based on morphology. Ph.D. Dissertation. Uppsala University. Uppsala, Sweden, 2001. 30. Rees, J.L. Skin cancer (Including Nevoid Basal Cell Carcinoma Syndrom). In B. Vogelstein and K.W. Kinzler, eds. The Genetic Basis ofHuman Cancer. 2nd edition. N.Y.: McGraw-Hill Companies, 2002. 31. Kamb, A., and Herlyn, M. Malignant melanoma. In B. Vogelstein and K.W. Kinzler, eds. The Genetic Basis of Human Cancer. 2 nd edition. N.Y.: McGraw-Hill Companies, 2002. 32. Friedman, R.J., Rigel, D.S., Kopf, A.W. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. CA CancerJ. Clin. 35: 130-151 (1985). 33. Takata, M. and Saida T. Early cancers of the skin: clinical, histopathological, and molecular characteristics. The Japan Society of Clinical Oncology 10: 391-397 (2005). 71 34. Gallagher, R.P., McLean, D.I., Yang, C.P., Coldman, A.J., Siver, H.K.B., Spinelli, J.J., and Beagrie, M. Anatomic distribution of acquired melanocytic nevi in white children: a comparison with melanoma - the Vancouver mole study. Arch Dermatol 126: 466-471 (1990). 35. Backvall, H., Wolf, 0., Hermelin, H., Weitzberg, E., and Ponten, F. The Density of epidermal p53 clones is higher adjacent to squamous cell carcinoma in comparison with basal cell carcinoma. British JournalofDermatology 150: 259-266 (2004). 36. Gailani, M.R., and Bale, A.E. Developmental genes and cancer: Role of patched in basal cell carcinoma of the skin. Journal of the National CancerInstitute 89: 11031108 (1997). 37. Gailani, M.R., Leffell D.J., Ziegler, A., Gross, E.G., Brash, D.E., Bale, A.E. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. Journalof the National CancerInstitute 88 (6): 349-354 (1996). 38. Knudson, Alfred G. Mutation and cancer: statistical study of retinoblastoma. Proc. NationalAcademy of Sciences USA 68: 820-823 (1971). 39. Knudson, Alfred G. Antioncogenes and human cancer. Proc. NationalAcademy of Sciences USA 90: 10914-10921 (1993). 40. Coller, H.A., Khrapko, K., Torres, A., Frampton, M.W., Utell, M. J., Thilly, W.G.. Mutational spectra of a 100-base pair mitochondrial DNA target sequence in bronchial epithelial cells: a comparison of smoking and nonsmoking twins. Cancer Research 58: 1268-77 (1998). 41. Coller, H.A., Khrapko, K., Bodyak, N.D., Nekhaeva, E., Herrero-Jimenez, P., Thilly, W.G.. High frequency of homoplasmic mitochondrial DNA mutations in human tumors can be explained without selection. Nature Genetics 28: 147-150 (2001). 42. Coller, H.A., Khrapko, K., Herrero-Jimenez, P., Vatland J.A., Li-Sucholeiki, X., Thilly, W.G. Clustering of mutant mitochondrial DNA copies suggests stem cells are common in human bronchial epithelium. Mutation Research 578: 256-271 (2005). 43. Wikonkal, N.M., and Brash, D.E. Ultraviolet radiation induced signature mutation in photocarcinogenesis. Journalof Investigative Dermatology Symposium Proceedings4: 6-10 (1999). 44. Nordling C. A new theory on cancer-inducing mechanism. British Journalof Cancer 7 (1): 68-72 (1953). 72 45. Armitage, P. and Doll, R. The age distribution of cancer and a multi-stage theory of carcinogenesis. British Journal of Cancer 8 (1): 1-12 (1954). 46. Armitage, P. and Doll, R. A two-stage theory of carcinogenesis in relation to the age distribution of human cancer. British Journalof Cancer 11: 161-169 (1957). 47. Moolgavkar, S.H., Kundson Jr., A.G. Mutation and cancer: a model for human carcinogenesis. Journalof the National CancerInstitute 66 (6): 1037-1052 (1981). 48. Brash, D.E., Zhang, W., Grossman, D., Takeuchi, S. Colonization of adjacent stem cell compartments by mutant keratinocytes. Seminars in CancerBiology 15: 97-102 (2005). 49. Brash, D.E., Seetharam, S., Kraemer, K.H., Seidman, M.M., and Bredberg, A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc. National Academy ofSciences USA 84: 3782-3786 (1987). 50. Ziegler, A., Leffell, D.J., Kunala, S., Sharma, H.W., Gailani, M., Simon, J.A., Halperin, A.J., Baden, H.P., Shapiro, P.E., Bale, A.E., and et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc. National Academy of Sciences USA 90:4216-4220 (1993). 51. Ponten, F., Berg, C., Ahmadian, A., Ren, Z., Nister, M., Lundeberg, J., Uhlen, M., and Ponten, J. Molecular pathology in basal cell cancer with p53 as a genetic marker. Oncogene 15: 1059-1067 (1997a). 52. Ren, Z., Ponten, F., Nister, M., and Ponten, J. Two distinct p53 immunohistochemical patterns in human squamous-cell skin cancer,(precursors and normal epidermis. Int. J. Cancer (Pred. Oncol.) 69: 174-179 (1996a). 53. Cairns, J. Mutation selection and the natural history of cancer. Nature 255: 197200 (1975). 54. Ouhtit, A., Ueda, M., Nakazawa, H., Ichihashi, M., Dumaz, N., Sarasin, A., and Yamasaki, H. Quantitative detection of ultraviolet-specific p53 mutations in normal skin from Japanese patients. CancerEpidemiology, Biomarkers & Prevention 6: 433-438 (1997). 55. Ouhtit, A., Nakazawa, H., Armstrong, B.K., Kricker, A., Tan, E., Yamasaki, H., and English, D.R. UV-Radiation-specific p53 mutation frequency in normal skin as a predictor of risk of basal cell carcinoma. Journalof the National CancerInstitute 90 (7): 523-531 (1998). 73 56. Ouhtit, A. and Ananthaswamy, H.N. A model for UV-inductio Journalof Biomedicine and Biotechnology 1: 5-6 (2001). n of skin cancer. 57. Nakazawa, H., English D., Randell, P.L., Nakazawa, K., Martel, N., Armstrong, B.K., and Yamasaki, H. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc. NationalAcademy ofSciences USA 91: 360-364 (1994). 59. Kawasaki, K., Suzuki, T., Ueda, M., Ishihashi, M., Reguer, G., and Yamasaki, H. CC to TT mutation in the mitochondrial DNA of normal skin: relationship to ultraviolet light exposure. Mutation Research 468: 35-43 (2000). 59. Berg, R.J.W., van Kranen, H.J., Rebei, H.G., de Vries, A., van Vloten,W.A., van Kreul, C.F., van der Leun, J.C., and de Gruul, F.R. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: Immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. PProc. NationalAcademy of Sciences USA 93: 274-278 (1996). 60. Brash, D.E., Wikonkal, N.M., Remenyik, E., van der Horst, G.T. , Friedberg, E..C., Cheo, D.L., van Steeg, H., Westerman, A., and van Kranen, H. The DNA damage signal for Mdm2 regulation, Trp53 induction, and sunburn cell formation in vivo originates from actively transcribed genes JournalofInvestigative Dermatology 117: 1234-1240 (2001). 61. Brash, D.E. and Havre, P.A. New careers for antioxidants. Proc. National Academy of Sciences 99: 13969-13971 (2002). 62. Brash, D.E. Roles of the transcription factor p53 in keratinocyte carcinomas. British JournalofDermatology 154 (Suppl. 1): 8-10 (2006). 63. Brash, D.E. and Haseltine, W.A. Photoreactivation of EscherichiaColi reverses umu C induction by UV Light. JournalofBacteriology 163 (2): 460-462 (1985). 64. Ponten, F., Williams C., Ling, G., Ahmadian, A., Nister, M., Lundeberg, J., Ponten, J., and Uhlen, M. Genomic analysis of single cells from human basal cell cancer using laser-assisted capture microscopy. Mutation Research 382 (1-2): 45-55 (1997b). 65 Ponten, F., Lindman, H., Bostrom, A., Berne, B., and Bergh, J. Induction of p53 expression in skin by radiotherapy and UV radiation: a randomized study. Journalof the National CancerInstitute, 93 (2): 128-133 (2001). 66. Gao Ling, Persson, A., Berne, B., Uhlen, M., Lundeberg, J., and Ponten, F. Persistent 74 P53 mutations in single cells from normal human skin. American Journalof Pathology 159 (4): 1247-1253 (2001b). 67. English, D.R., Armstrong, B.K., Kricker, A., Winter, M.G., Heenan, P.J., and Randell, P.L. Demographic characteristics, pigmentary and cutaneous risk factors for squamous cell carcinoma of the skin: A case-control study. International Journalof Cancer76: 628-634 (1998). 68. English, D.R., Milne, E., Simpson, J.A. Ultraviolet radiation at places of residence and the development of melanocytic nevi in children (Australia). Cancer Causes and Control 17: 103-107 (2006). 69. Marks, R. The relationship of basal cell carcinomas and squamous cell carcinomas to solar keratoses. Arch Dermatol 124: 1039-1042 (1988). 70. Kricker, A., Armstrong, B.K., English, D.R., and Heenan, P.J. A dose-response curve for sun exposure and basal cell carcinoma. InternationalJournalof Cancer 60: 482-488 (1995). 71. Kricker, A., Armstrong, B.K., English, D.R., and Heenan, P.J. Does intermittent sun exposure cause basal cell carcinoma? A case-control study in western Austrialia. InternationalJournalof Cancer 60: 489-494 (1995). 72. Hedrum, A., Ponten, F., Ren, Z., Lundeberg, J., Ponten, J., and Uhlen, M. Sequence-based analysis of the human p53 gene based on microdissection of tumor biopsy samples. BioTechniques 17(1):118-129 (1994). 73. Kreamer, Kenneth H. Sunlight and skin cancer: another link revealed. Proc. NationalAcademy ofScience 94: 11-14 (1997). 74. Zhang, W., Remenyik, E., Zelterman, D., Brash, D.E., and Wikonkal, N.M. Escaping the stem cell compartment: Sustained UVB exposure allows p53-mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proc. NationalAcademy ofSciences USA 98 (24): 13948-13953 (2001). 75. Zhang, W., Hanks, A.N., Boucher, K., Florell, S.R., Allen, S.M., Alexander, A., Brash, D.E., and Grossman D. UVB-induced apoptosis drives clonal expansion during skin tumor development. Carcinogenesis26 (1): 249-257 (2005). 76. Birch-Machin, M.A., Tindall, M., Turner, R., Haldane, F., and Rees, J.L. Mitochondrial DNA deletions in human skin reflect photo - rather than chronologic aging. The Societyfor Investigative Dermatology, 110 (2):149-152 (1998). 75