Solution
advertisement
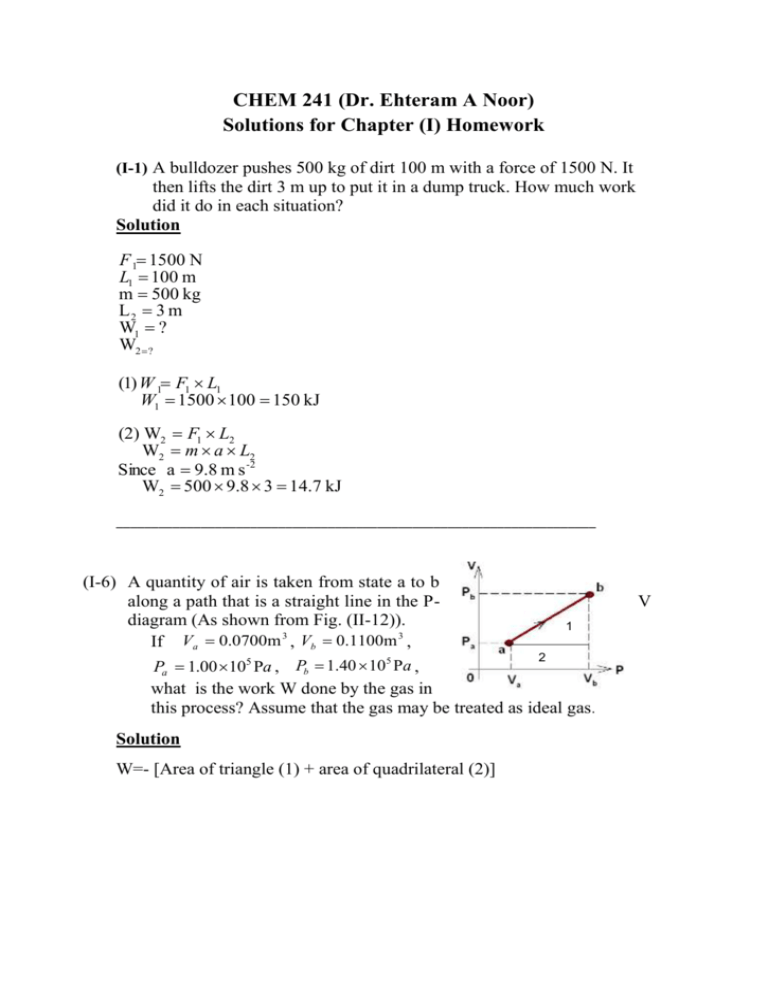
CHEM 241 (Dr. Ehteram A Noor) Solutions for Chapter (I) Homework (I-1) A bulldozer pushes 500 kg of dirt 100 m with a force of 1500 N. It then lifts the dirt 3 m up to put it in a dump truck. How much work did it do in each situation? Solution F 1 1500 N L1 100 m m 500 kg L2 3 m W1 ? W2 ? (1) W 1 F1 L1 W1 1500 100 150 kJ (2) W2 F1 L2 W2 m a L2 Since a 9.8 m s -2 W2 500 9.8 3 14.7 kJ ____________________________________________________________________ (I-6) A quantity of air is taken from state a to b along a path that is a straight line in the Pdiagram (As shown from Fig. (II-12)). 1 If Va 0.0700m 3 , Vb 0.1100m 3 , 2 5 Pa 1.00 105 Pa , Pb 1.40 10 Pa , what is the work W done by the gas in this process? Assume that the gas may be treated as ideal gas . Solution W=- [Area of triangle (1) + area of quadrilateral (2)] V 1 ( Pb Pa )(Vb Va ) 2 Area of quadrilate ral Pa (Vb Va ) 1 W [ ( Pb Pa )(Vb Va ) Pa (Vb Va )] 2 1 1 W (Vb Va )( Pb P a Pa ) 2 2 1 W (Vb Va )( Pb Pa ) Area of trapezoid 2 The negative signe of work indicates that the work is done by the system Area of triangle Trapezoid ()شبه منحرف W 1 / 2 105 (0.11 0.07)(1 1.4) W 4.8kJ ____________________________________________________________________ Solutions for Chapter (II) Homework (II-1) When a quantity of monoatomic ideal gas expands at constant pressure of 4.0 10 4 Pa , the volume of the gas increases from 2.0 10 3 m 3 to 8.0 10 1 m 3 . What is the work done by the gas? Solution Pext 4 10 4 Pa V1 2 10 3 m 3 V2 8 10 1 m3 W PV W 4.0 10 4 (8 10 1 2.0 10 3 ) W 31.92 kJ ____________________________________________________________________ (II-2) One mole of an ideal gas at 300 K is allowed to expand isothermally from a volume of 1.00 L to a volume of 5L. Find the work done by the gas and the heat energy absorbed from the surroundings. Solution T cons tan t 300 K V1 1L V2 5L W nRT ln W ? q? V2 V1 W 1 8.314 300 ln 5 4.014 kJ 1 E 0 E q W q W q (4.014) 4.014 kJ ____________________________________________________________________ (II-8) Calculate q, W , E and H if 1.00 mole of an ideal gas with 3 C V R undergoes a reversible adiabatic expansion from an 2 3 initial volume Vi 5.25m 3 to final volume V f 25.5m . The initial temperature is 300 K. Solution Since the process is adiabatic: Vi 5.25 m3 q0 V f 25.5 m3 H 0 Ti 300 K E W W nCV (T2 T1 ) From the adiabatic relations: TiVi 1 T f V f 1 1 R 2 R 0.67 Cv 3R 300 5.250.67 T f 25.50.67 T f 104.05 K 3 8.314 (104.05 300) 2 W 2.444kJ E 2.444kJ W 1 ____________________________________________________________________ (II-9) One mole of He in state defined by Ti 300 K and Pi 23.0 atm undergoes an isothermal reversible expansion until Pf 2.5 atm . Calculate W assuming the gas is described by the ideal gas law. W nRT ln P1 P2 W 1 8.314 300 ln 23 55.54 kJ 2.5 ____________________________________________________________________ (II-13) A-A body of air which occupies 1000 cm3 at 30 oC and 76 cmHg, expands isothermally and reversibly to a volume 2000 cm3. What is (a) the final pressure? (b) the work done? (c) the heat absorbed? Solution From Boyl , s law : P1V1 P2V2 PV P2 1 1 V2 76 P1 1 atm V1 1L 76 1 1 P2 0.5 atm 2 V V W nRT ln 2 P1V1 ln 2 V1 V1 W 1 1 ln 2 0.693 L atm L atm 101.3 J W 70.20 J For isothermal process : E 0 q -W q 70.20 J V2 2 L











