What are common results of heat transfer? 3. Mechanical work.
advertisement
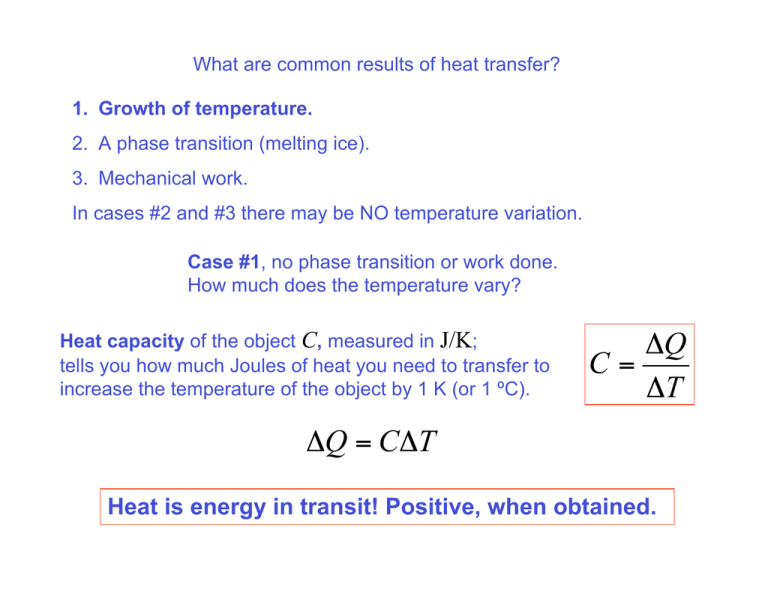
What are common results of heat transfer? 1. Growth of temperature. 2. A phase transition (melting ice). 3. Mechanical work. In cases #2 and #3 there may be NO temperature variation. Case #1, no phase transition or work done. How much does the temperature vary? Heat capacity of the object C, measured in J/K; tells you how much Joules of heat you need to transfer to increase the temperature of the object by 1 K (or 1 ºC). !Q C= !T !Q = C!T Heat is energy in transit! Positive, when obtained. Heat capacity of the object C, measured in J/K; tells you how much Joules of heat you need to transfer to increase the temperature of the object by 1 K (or 1 ºC). !Q C= !T The term “heat capacity” is used for historic reasons and is confusing! It sounds as if the object “contains heat”, whereas by definition heat is energy in transit. An object contains some internal energy (not heat!) and its temperature is a measure of this internal energy. An analogy from mechanics: you do some work on an object and the object gains the same amount of energy (kinetic, potential) as a result. Mechanical work also relates to a process, not state. !K + mg!h = W Heat capacity is an extensive (integral) parameter: When you bring two objects together, heat capacity of the system of the two objects becomes the sum of the two individual heat capacities. It is convenient to introduce specific heat, c, which is heat capacity of a material per unit mass. Specific heat is measured in J/(K⋅kg). C = cm C !Q c= = m m!T !Q = cm!T Heat capacity of a water balloon is large due to both high specific heat and large mass of the water inside the balloon. Specific heat, c, is heat capacity of a material per unit mass. Specific heat is measured in J/(K⋅kg). !Q = cm!T C !Q c= = m m!T The equilibrium temperature. Situation: two objects with different temperatures, T1 and T2, are brought in a thermal contact and reach thermal equilibrium at a temperature T after a while. Heat ΔQ1 is transferred to Object 1; heat ΔQ2 is transferred to Object 2. By energy conservation: !Q1 + !Q2 = 0 !Q1 = "!Q2 By definition of heat capacity and specific heat: "Q1 = C1"T1 = m1c1"T1 = m1c1 (T ! T1 ) "Q2 = C2 "T2 = m2 c2 "T2 = m2 c2 (T ! T2 ) m1c1 (T ! T1 ) + m2 c2 (T ! T2 ) = 0 m1c1T1 + m2 c2T2 C1T1 + C2T2 T= = m1c1 + m2 c2 C1 + C2 Heat transfer 1.Conduction. 2. Convection 3. Radiation Setting: A rectangular slab of thickness Δx and with an area A. The front side of the slab is at a temperature T; the back side has a somewhat different temperature, T+ΔT. We are trying to calculate the heatflow rate, the amount of heat flowing through the slab per unit time, H = ΔQ/Δt. We expect H to be proportional to the area of the slab, the temperature difference, ΔT, between the back and the front and inversely proportional to the thickness of the slab, Δx. H should also somehow depend of properties of the material the slab is made of… Bringing all the parts together: !T H = "kA !x The coefficient k reflects specific properties of the material of the slab and is called thermal conductivity H = ΔQ/Δt – heat-flow rate is measured in Joules/second, J/s, or Watts, W. Thermal conductivity, k, is measured in W/(m⋅K). Thermal conductivities of different materials. Best heat conductor – Copper; use it when you build heat sink, as a material for pipes in your cooling system, a radiator. Worst heat conductors are the best insulating materials – air, fiberglass (layers in the walls of houses in cold regions), styrofoam (cups for your hot coffee). Heat-flow rate equation !T !T H = " kA =" !x !x /(kA) Is similar to the Ohm’s law: V I= R The current, I = Δq/Δt, amount of charge per unit time, is analogous to the heat-flow rate, H = ΔQ/Δt. The voltage, V, the factor driving the electric current, is analogous to temperature difference, ΔT. Continuing the analogy: electric resistance, R, is analogous to Thermal resistance is introduced as Thermal resistance is introduced as Heat-flow rate equation !T H =" R A composite slab is analogous to two resistors connected in a series. Heat-flow rate "Ttotal T3 ! T1 H =! = Rtotal R1 + R2 Reducing heat-flow rate for better thermal insulation. Better thermal insulation… red or blue? Licking an ice cream, which is frozen, seems to be OK… What about the door handles, when it is freezing outside? Which one would you prefer? Cold metals are especially bad because of their high… thermal conductivity In order to keep your tongue above 0 °C you basically have to heat the whole piece of metal… Otherwise… Thermal conductivities of different materials. Sample problem A home heating system supplies heat at the maximum rate of 40 kW. If the house loses 1.1 kW for each °C between inside and outside, what is the minimal outdoor temperature for which the heating system can maintain 20 °C inside? Solution: the lowest temperature outside for which the heating system can maintain 20 °C inside corresponds to the case, when the maximal power of the heater, Hmax=40 kW all flows outside because of temperature difference. H out 1.1kW = !T = H max = 40 kW 1°C 40 kW !T = °C = 36 °C 1.1 kW Tout = Tin ! "T = !16 °C Convection - Heat transfer in a gas or liquid by the circulation of currents from one region to another. Can be forced or spontaneous (natural). Hot and cold liquid is brought in a thermal contact; it reduces the distance across which the conduction occurs and increases the contact area. !T H = " kA !x






