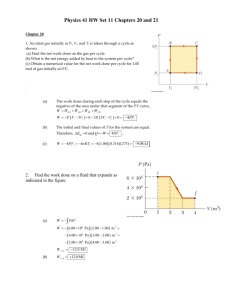
PHYSICS 140A : ASSIGNMENT #2 SOLUTIONS (a) As we know "
advertisement
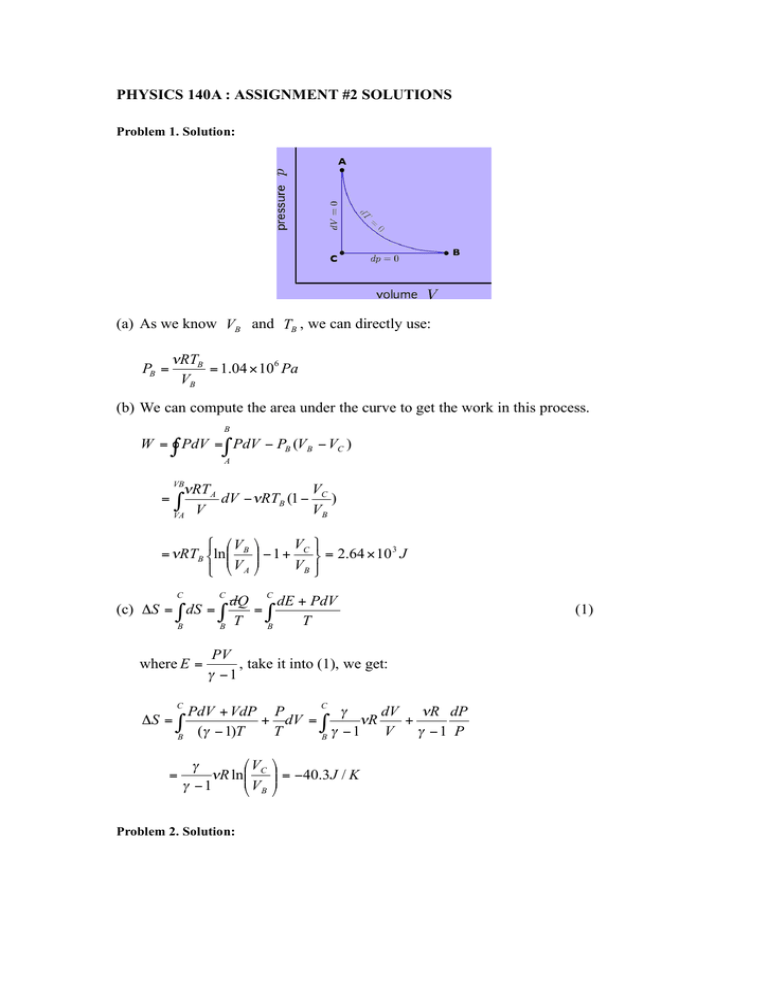
PHYSICS 140A : ASSIGNMENT #2 SOLUTIONS Problem 1. Solution: (a) As we know VB and TB , we can directly use: "RTB = 1.04 ! 106 Pa VB PB = (b) We can compute the area under the curve to get the work in this process. B W = " PdV = " PdV ! PB (VB ! VC ) A VB = V #RT A dV ! #RTB (1 ! C ) V VB VA " ' .V + V $ = /RTB &ln,, B )) ( 1 + C # = 2.64 ! 10 3 J VB " % - VA * C C C dQ dE + PdV (c) "S = ! dS = ! =! T T B B B where E = PV , take it into (1), we get: " !1 C C PdV + VdP P $ dV %R dP + dV = ! %R + ($ " 1)T T $ "1 V $ "1 P B B #S = ! = 'V ) (R ln%% C ) !1 & VB Problem 2. Solution: $ "" = !40.3 J / K # (1) First of all, let’s consider the work during this period, i.e. S ABCD B C D A W = S ABCD = ! PdV + ! PdV + ! PdV + ! PdV A B C D B D PAV A# PAVD# = ! # dV + ! # dV + PA (V A " VD ) A V C V = ! 1 PA (V A " VD ) " PAVB1"! (V A! " VD! ) ! "1 ! "1 Secondly, in this period, the ideal gas absorb heat during D->A, therefore: A A A ' PV $ ) "" + PA (V A ! VD ) = Q = ( dQ = ( dE + PdV = ( d %% PA (V A ! VD ) ) !1# ) !1 D D D & Finally, we get: W 1 r 1!" ( s " ! 1) #= = 1! , where s and r have been defined in the problem. Q " s !1 'V Otto cycle has the efficiency of ) = 1 ! %% min & Vmax $ "" # ( !1 If r=18, s=2, ! Diesel = 81% < ! Otta = 85% , however an optimized Otto Cycle has the parameter of r=8, ! max ofOtta = 75% , which is less than the efficiency in Diesel cycle. Problem 3. Solution: (a) The unit of S is J/K, V is m 3 and E is J, therefore, a should has the unit of: m6 K 5 / J 4 . aS 5 5aS 4 2aS 5 (b) E = 2 2 " dE = 2 2 dS ! 3 2 dV V N V N V N (1) On the other hand, we know: dE = TdS ! PdV Compare (1) and (2), we can easily get the relation: T= (2) 5aS 4 2aS 5 P = , V 2N 2 V 3N 2 Then we can get the equation of state: &V # P$ ! %N" 1/ 2 5 5 / 4 a 1 / 4 = 2T 5 / 4 (c) As a isotherm process, the equation of state should be: PV 1 / 2 = C = const. Therefore, the work in this isotherm process is: C W = ! PdV = ! 1 / 2 dV = 2C (V f1 / 2 " Vi1 / 2 ) V (d) Always be aware of the difference between isotherm process and adiabatic process! First, let’s rewrite the equation of state as: 16 P 4V 2 a = N 2T 5 3125 We can then get the differential relation: 16 2 4 4 P 3V 2 adP + 2 P 4VadV = N T dT (1) 625 As adiabatic process, dE = ! PdV (2) aS 5 (VN )1 / 2 5 / 4 Where E = 2 2 = 5 / 4 1 / 4 T , and P is determined by the equation of state V N 5 a as above. The (2) can give us the relation of dV and dT, dT = ! Invoking this relation to (1), we can get: ( 1 & 'V # 1 = $ ! V % 'P " dQ =0 3P Problem 4. Solution: (a) Similar as the problem 3, dE = TdS ! PdV !" ( )E % T =& # = 0 kB ' )S $V ! !S (N% ) & # exp( Nk B 'V $ 2T dV V ( )E % (N% P = *& # = !" 0 & # ' )V $ S 'V $ 1+! exp( !S ) Nk B Therefore, PV = NkT = !RT . ! This system obeys the ideal gas law. (b) As for adiabatic process, dE = dQ ! PdV = ! PdV = ! In addiation, we can rewrite E = Therefore, dE = Nk B T dV V Nk B T by using the relation in (a). ! Nk B dT , invoking this expression into (1): ! Nk B Nk T dT = ! B dV ! TV " = const. " V (c) From the derivation above, we can get cV = 1 ( )E % R & # = " ' )T $V ! 1+! ' (V $ c P = cV + p% R " = ! & (T # p Problem 5. Solution: (a) The volume relation should be the same as the normal Carot Cycle. i.e. dQ ! T = 0 , where only A->B and C->D contribute dQ. Thus, (1) (b) Let’s analysis the work in details. AA’: Q AA' = W AA' = 0 &V # A’B: Q A'B = W A'B = nRT2 ln$$ B !! % V A' " BC: Q BC = 0 ; W BC = nR(T2 ! T1 ) " !1 & VC % VD CD: QCD = WCD = ' nRT1 ln$$ DA: Q DA = 0 ; W DA = ! # &V # !! = 'nRT1 ln$$ B !! " % VA " nR(T2 ! T1 ) " !1 The total work in this cycle is: &V # &V # W = W AA' + W A'B + WBC + WCD + WDA = nRT2 ln$$ B !! ' nRT1 ln$$ B !! % V A' " % VA " By using the defined parameter x and r, we can get: W = nR(T2 ! T1 ) ln r ! nRT2 ln (1 + (r ! 1) x ) (c) Let W = nR (T2 ! T1 ) ln r ! nRT2 ln (1 + ( r ! 1) x ) = 0 , we can solve this problem, and the answer is x = r 1"T1 / T2 " 1 ! 0.26 r "1 & # & # VB r !! = nRT2 ln$$ !! , therefore (d) Q A'B = nRT2 ln$$ V + x ( V ' V ) 1 + x ( r ' 1 ) % " B A " % A "= T T W ln r = 1! 1 < 1! 1 Q A' B T2 ln r ! ln(1 + (r ! 1) x) T2