Massachusetts Institute of Technology Organic Chemistry 5.512 Problem Set 7
advertisement

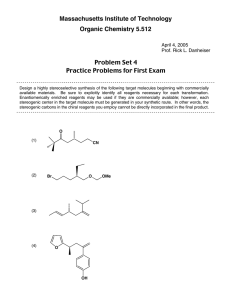
Massachusetts Institute of Technology Organic Chemistry 5.512 May 4, 2005 Prof. Rick L. Danheiser Problem Set 7 Stereocontrolled Synthesis of Acyclic Molecules Review Problems for Second Exam Design a highly stereoselective synthesis of the following target molecules beginning with commercially available materials. Be sure to explicitly identify all reagents necessary for each transformation. Enantiomerically enriched reagents may be used if they are commercially available; however, with the exception of the two compounds shown below, each stereogenic center in the target molecule must be generated in your synthetic route. In other words, the stereogenic carbons in the chiral reagents you employ cannot be directly incorporated in the final product. The exceptions are (S) and (R) methyl 3hydroxy-2-methylpropionate, which are commercially available and have been widely employed in total synthesis. HO HO CO2Me CO2Me A stereoselective synthesis of each of these target molecules has been reported in the literature and a reference for each synthesis is provided at the end as an appendix. In addition, an outline of these literature syntheses will be posted on the MIT server for your reference. Note, however, that the original route to each molecule may not be the optimal approach, especially in view of new methods that have been reported since the literature route was developed! To derive maximum benefit from these problems, I recommend that for each target you consider all possible synthetic routes that can be envisioned based on the methods and strategies studied in 5.512, and then critically compare your viable approaches and decide which would be most practical and efficient. (1) O O OHC OH (2) t-BuMe2SiO O OH (3) Br Br O O (4) OH O O OH (5) CO2Et Ph3CO (6) t-BuMe2SiO OBn (7) EtO2C OBn OBn (8) Br O OEt t-BuMe2SiO (9) OH OH (10) OH CO2H i-Pr3SiO (11) OH BnO OH OBn OMe (12) OMe OSit-BuMe2 OH S S OHC (13) t-BuMe2SiO OH CO2Me (14) MeO MeO (15) MeO2C CO2Me OH SiMe3 Br (16) OH CO2Me (17) BnO t-BuMe2SiO OSit-BuMe2 OSit-BuMe2 t-BuMe2SiO OH O (18) N OMe (19) O t-BuPh2SiO OMe Me O OH O (20) O BnO OSiMe3 OH (21) O OMe OH O OH OMe The following problems are taken from the second exam in previous years. The instructions were identical to those on page 1. (22) CO2Me BnO t-BuMe2SiO OH (23) OSiMe3 See total synthesis of roflamycoin, S. Rychnovsky !J. Am. Chem. Soc. 1994, 116, 175 OH See synthesis of C(1)-C(14) fragment of callipeltoside A, T. R. Hoye !Org. Lett. 1999, 1, 169 O OMe BnO (24) OSit-BuMe2 EtO2C OBn (25) Intermediate for synthesis of epothilone A; see, for example J. S. Panek !Org. Lett. 2000, 2, 2575 CO2Et O H N Ph Ph O O t-BuMe2SiO OH O (26) Et3Si OCOt-Bu O See total synthesis of sanglifehrin A, K. C. Nicolaou et al.! J. Am. Chem. Soc. 2000, 122, 3830 OSit-BuMe2 O (27) O See synthetic studies on miyakolide, S. Masamune et al. J. Org. Chem. 1997, 62, 8978 OBn CH3 O HN (28) O H OH AnCH2O2C An = p-OMeC6H4 OH See J. E. Baldwin et al. Tetrahedron Lett. 1987, 28, 3605 O OH O (29) H OSit-BuMe2 OBn See total synthesis of resiniferatoxin, P. A. Wender et al.! J. Am. Chem. Soc. 1997, 119, 12976 (30) O O t-BuMe2SiO OH OH (31) OBn See synthetic studies on spongistatin 1, M. T. Crimmins et al. Org. Lett. 2001, 3, 949 OPMB OAc See total synthesis of (+)-13-deoxytedanolide, A. B. Smith et al. J. Am. Chem. Soc. 2003, 125, 350 (32) t-BuMe2SiO (33) OH H2N OBn