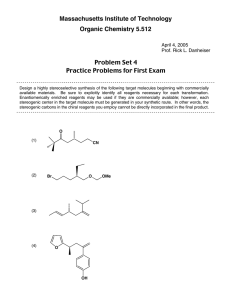
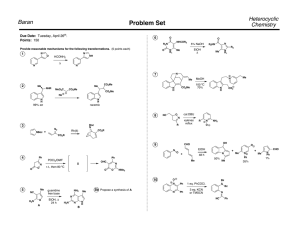
B4M1 studymeeting #10 answer Zhai Pericyclic reaction 1. One step in the synthesis of periplanone B, the chapter-opening molecule, involved anionic oxyCope rearrangement of the following unsaturated alcohol. Draw the product that results after protonation of the intermediate enolate. 2. Classify each pericyclic reaction as an electrocyclic reaction, cycloaddition, or sigmatropic rearrangement. Indicate whether the stereochemistry is conrotatory, disrotatory, suprafacial, or antarafacial. 3. What type of pericyclic reaction is illustrated in each reaction? 4. Draw a stepwise, detailed mechanism for the following reaction. 5. Provide a mechanism for each reaction below and explain stereochemistry if needed. kinetic product of the reaction 6.Provide a mechanism for each reaction below and explain stereochemistry if needed. 7.Provide a mechanism for each reaction below and explain stereochemistry if needed. 8.Provide a mechanism for each reaction below and explain stereochemistry if needed. 9.Provide a mechanism for each reaction below and explain stereochemistry if needed. 10.Provide a mechanism for each reaction below and explain stereochemistry if needed. 11.Provide a mechanism for each reaction below and explain stereochemistry if needed. 12.Provide a mechanism for each reaction below and explain stereochemistry if needed. 13.Provide a mechanism for each reaction below and explain stereochemistry if needed. 14.Total synthesis A C B D E 1)Mechanism of A Pictet-Spengler reaction CO2Me N CO2Me N BnN BnHN HO2C O CO2H O CO2Me NBn N BnN HO O CO2Me N CO2H CO2H OH2 N CO2Me NBn N H CO2Me NBn CO2H CO2H CH2O2H (2)Mechanism of B -(1) Dieckmann condensation NaH CO2Me N N Bn N CO2Me CO2Me N Bn H OH N MeOH,PhMe Bn O CO2Me N N Bn OMe N O O OMe OMe H N Bn N OH CO2Me N Bn N O CO2Me (3)Mechanism of C OH Bn N Bn N N N MeO MeO Me MeO OMe H MeO Me MeO Bn N N N N OMe H Bn N Bn N O O O CO2Me OMe MeO Me H (4)Mechanism of D-(2) Swern oxidation O Cl Cl Cl Cl O S O O R O O CO2,CO R O S H S Cl OH Et3N H H R O H S R O R SH (5)Mechanism of E Knoevenagel pyridine synthesis O HN NH2OH H O OH OH HN O N OH O N OH N OH2 OH N OH2 H H N O S