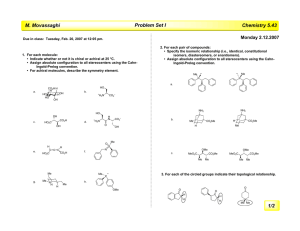
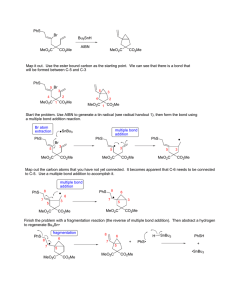
Heterocyclic Chemistry Problem Set
advertisement

Baran Heterocyclic Chemistry Problem Set Due Date: Tuesday, April 26th. Points: 150 6 O O NHCOR3 R2N Provide reasonable mechanisms for the following transformations. (5 points each) N 1 O N O EtOH Δ Me Δ N Me Me NHR R3 N R1 Me N 7 2 N R2HN NH HCONH2 N N R1 5% NaOH Me MeO2C CO2Me CO2Me CO2Me 100 ºC 70% CO2Me N H Me N MeOH N H CO2 Na N H N H 99% ee racemic 8 NBoc + N N2 xylenes reflux R Boc N 3 cat DBU O NC R N NH2 O CO2R Rh(II) CO2R 9 N 4 Ph N O OHC X O guanidine free base NH2 Me N H2N O A 48 h 50% O EtOH, Δ 24 h H2N N Bz Ph 1 eq. PhCOCl, N Ph 3 eq. KCN or TMSCN N NC N H N B Me N Ph Me NMe2 5b Propose a synthesis of A O + N N N NC + r.t., then 80 ºC O Me O Me 10 5 EtOH Ph POCl3/DMF O OH CHO Bz Ph 35% CHO + Me N Ph 1% Baran Heterocyclic Chemistry Problem Set 11 N O CN CHO NaOH, N MeOH 58% O C 11b Propose a synthesis of C Provide simple syntheses for the following compounds. (5 points each) (You may not use Pd or other similar metals that achieve cross-coupling.) Hint: Most syntheses possible in 1–3 steps. O 3 D 1 CO2Me N F 2 Ph 12 Me O Me pulegone NH2 toluene, reflux, NH2 4 days 68% + Me Me N CO2Me N H N CO2H N Me N H NHMe Me F 4 Br 5 N Me Advanced problems in heterocyclic chemistry (10 points each). 1 Treatment of quinolizinium bromide with two equivalents of piperidine gives a high yield of a product E, C14H18N2. Reaction of E with phenacyl bromide followed by quenching of the reaction mixture with water gives a product F which was originally claimed to be 3benzoyl-2-vinylindolizine. Subsequent reinvestigation of the structure of F, however, showed that it was in fact 3-(2-phenyl-1-indolizinyl)prop-2-ene-1-al. Deduce the structure of E and give a reasonable explanation for the conversion of E into F. CHO Ph N 6 N H N O Me Me N 9 Br CONHBn 7 8 Ph Me N N Me Ph N F N N H Me Br HO2C 10 COMe WolffKishner N 11 CO2H 12 N O N Br O No Desired Product, obtained X in 62% yield Me Me Me Provide the mechanism of formation and structure of X: 1H O O F Cl 2 OTBS F NMR: δ 9.26 (s, 1H), 8.01 (brs, 1H, D20 exchangeable), 7.75 (s, 1H), 2.59 (q, 2H), 2.43–2.20 (m, 4H), 2.35 (s, 3H), 1.56 (m, 4H), 1.24 (t, 3H). 15 Me 13 O Br N H S 14 S MeO2C Me NO2 H CO2Me N H Me