Chemistry 634: Advanced Organic Chemistry – Synthesis and Reactivity
advertisement
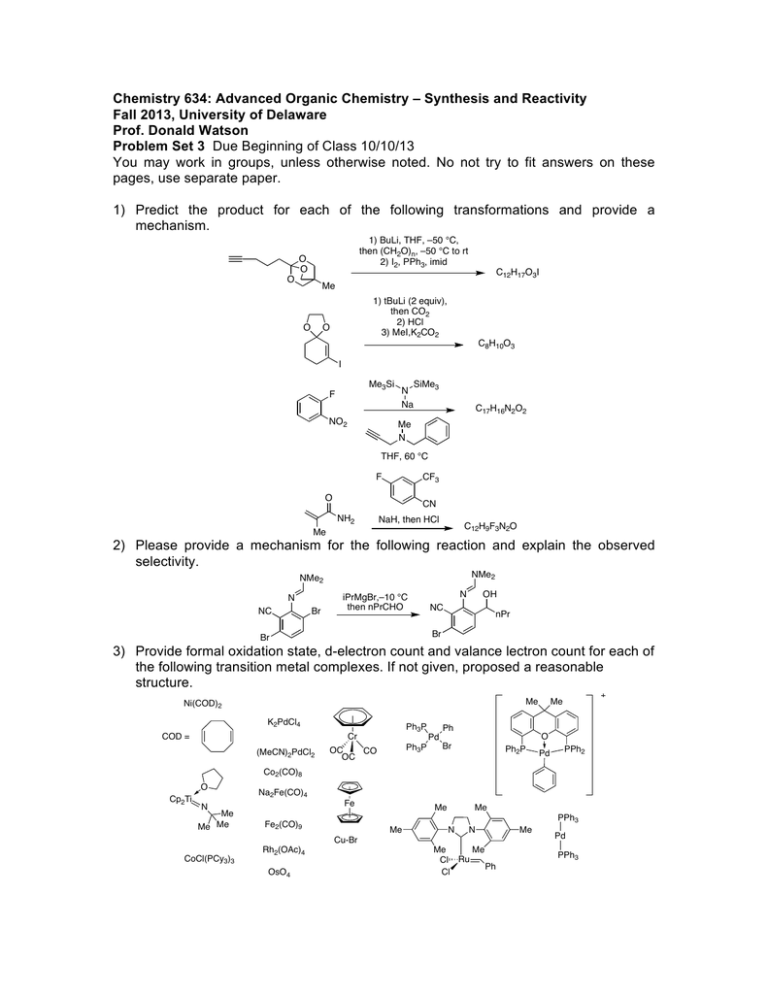
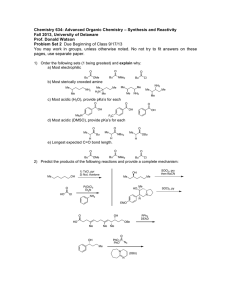
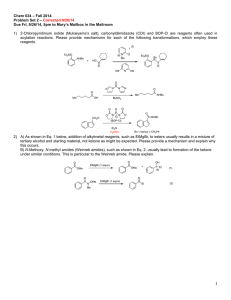
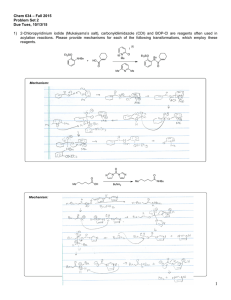
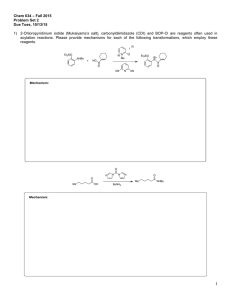
Chemistry 634: Advanced Organic Chemistry – Synthesis and Reactivity Fall 2013, University of Delaware Prof. Donald Watson Problem Set 3 Due Beginning of Class 10/10/13 You may work in groups, unless otherwise noted. No not try to fit answers on these pages, use separate paper. 1) Predict the product for each of the following transformations and provide a mechanism. 1) BuLi, THF, –50 °C, then (CH2O)n, –50 °C to rt 2) I2, PPh3, imid O O O C12H17O3I Me O 1) tBuLi (2 equiv), then CO2 2) HCl 3) MeI,K2CO2 O C8H10O3 I Me3Si F NO2 N Na SiMe3 C17H16N2O2 Me N THF, 60 °C F CF3 O CN NH2 NaH, then HCl C12H9F3N2O Me 2) Please provide a mechanism for the following reaction and explain the observed selectivity. NMe2 NMe2 N NC Br iPrMgBr,–10 °C then nPrCHO OH N NC nPr Br Br 3) Provide formal oxidation state, d-electron count and valance lectron count for each of the following transition metal complexes. If not given, proposed a reasonable structure. Me Ni(COD)2 K2PdCl4 COD = Ph3P Cr (MeCN)2PdCl2 Ph3P OC CO OC Ph Me O Pd Br Ph2P Pd PPh2 Co2(CO)8 O Cp2Ti Na2Fe(CO)4 Fe N Me Me Me Me Me PPh3 Fe2(CO)9 Me N N Cu-Br CoCl(PCy3)3 Rh2(OAc)4 OsO4 Me Me Cl Ru Ph Cl Me Pd PPh3 4) Predict the product for the following reactions. If a single enantiomer of product is expected, state so, but do not worry about which enantiomer is formed from the enantiomer of catalyst used. : Pd2(dba)3 SPhos K3PO4 Cl B(OH)2 + Me Me O Pd2(dba)3 SPhos NBn I I Pd(PPh3)4 + SnBu3 H H Me ClMg Bu EtO2C I + Ph B O O Pd(PPh3)4 Pd(PPh3)4 + EtONa Br ZnBr O NC Pd(OAc)2/1 NaOtBu Cl OTf Me N + PdCl2, PPh3 Et3N C8H17 NH2 N O OAc N H Ph I CO2Et Pd(PPh3)4, NaH CO2Me CO2Me CuI (cat), K3PO4 NHMe MeHN (cat) O NaCH(CO2Me)2 cat. [Ir(COD)Cl]2 cat 3 OAc H PhCCH, 4 Et3N, Zn(OTf)2 Ph PtBu2 Fe Me PCy2 1 CyPF-tBu Josiphos PCy2 OMe MeO SPhos Me P N O Me Ph Me O 3 NMe2 HO 4 5) Two related reactions from the Grigg group are shown below. A) Please provide a mechanism for each reaction. B) Rationalize the observed relative stereochemistry for each transformation by providing 3-D drawings of the possible completing transition states and providing arguments for why one maybe preferred. C) Finally, as you will see from the correct mechanisms for each, the order of events differs greatly with respect to CO in each of these reactions. Please discuss what factors might influence how and when CO undergoes reaction in these types of cyclizations. O 10% Pd(OAc)2 20 mol% P(2-furyl)3 CO, NaBPh4 anisole, 120 °C O Ph 84% O I TfO 10 mol% Pd(OAc)2 20 mol% PR3 Bu3N, CO morpholine 1,1-dimethylallene MeCN, 70 °C Me CO2R Me O H Me Me O N O 60% CO2R For the following questions, please work alone. You may use Reaxys, Scifinder or Web of Knowledge for these problems. 6) Using Reaxys or Scifinder, please identify up to 3 major US commercial suppliers (ie Aldrich, Acros, Strem, etc) for each of the following. If fewer suppliers exist, please state so. (Please see note on the course Syllabus regarding commercial sources). OMe Cl Li N iPr 2·BF4 Bu2Mg N F 7) O MeO iPr PCy2 iPr O iPr Please identify at least one paper that provides indicated spectral data in the specified solvent. the 1H NMR spectra in CDCl3 for the following compounds. O PPh3 OMe 1H in CDCl3 31P in d6-acetone 8) As you may appreciate, the chemical literature related to organic synthesis dates back to around 1850, and (as the law of physics have not change significantly since then) procedures dating back that far can still be very useful. Early organic chemistry, however, was mostly published in German. One also finds useful preparations reported in French, Russian, Japanese, etc. A good organic chemist does not ignore these papers, even if they are not read the language in which they were published. As the previous problem points out, Ruppert’s reagent (TMSCF3) and its derivatives are becoming important reagents in cross-coupling chemistry for preparing fluorinated organic molecules. Working alone: Please look up Ruppert’s original procedure for the preparation of TMSCF3 (Tetrahedron Lett. 1984, 25, 2195). Please give a detailed description of how the reagents is prepared. Include solvent and temperature, as well as workup conditions. What yield was reported and what characterization was provided? Report the NMR data given, and state what reference was used for each spectrum. Also please describe what side reactions were found in this reaction and what steps were taken to overcome them. Please type/chemdraw your answers for this question and hand in the answer separately from the rest of the problem set. You must work alone for this question, but you are allowed to use modern language tools, such as dictionaries, BabelFish or Google Translate. Please see me if you have questions.
![[Rh(acac)(CO)(PPh3)]: an Experimental and Theoretical Study of the](http://s3.studylib.net/store/data/007302827_1-767d92e522279b6bdb984486560992de-300x300.png)