Chem 634 – Fall 2015 Problem Set 2 Due Tues, 10/13/15
advertisement
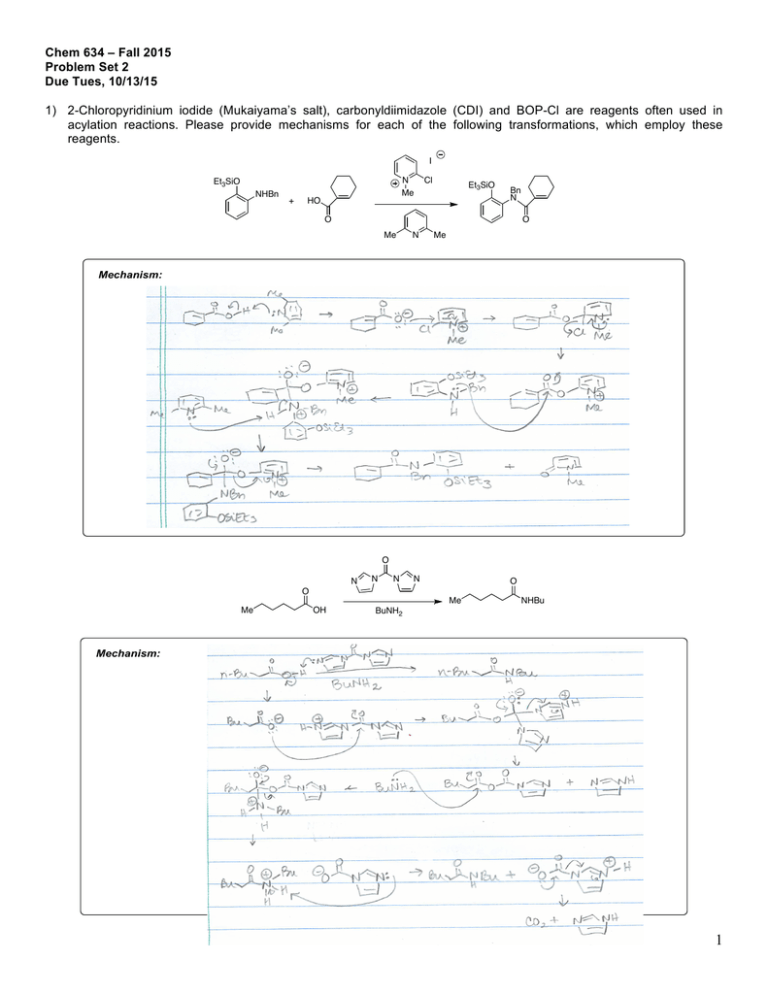
Chem 634 – Fall 2015 Problem Set 2 Due Tues, 10/13/15 1) 2-Chloropyridinium iodide (Mukaiyama’s salt), carbonyldiimidazole (CDI) and BOP-Cl are reagents often used in acylation reactions. Please provide mechanisms for each of the following transformations, which employ these reagents. I N Me Et3SiO NHBn + HO Cl Et3SiO Bn N O O Me Mechanism: N Me O N N N N O O Me Me OH O CO2H O O O P N N O Cl (BOP-Cl) Et3N H2NBn + HO O NHBn Bn = benzyl = CH2Ph I N Me Et3SiO NHBn NHBu BuNH2 Cl Et3SiO Bn N O O Me N Me O N N N N O O Me Me OH O Mechanism: CO2H NHBu BuNH2 O O O P N N O Cl (BOP-Cl) Et3N H2NBn O NHBn Bn = benzyl = CH2Ph 1 N N N N O O Me Me OH O CO2H NHBu BuNH2 O O O P N N O Cl (BOP-Cl) O NHBn Et3N H2NBn Bn = benzyl = CH2Ph Mechanism: 2) As shown in Eq. 1 below, addition of alkylmetal reagents, such as EtMgBr, to esters usually results in a mixture of tertiary alcohol and starting material, not ketone as might be expected. N-Methoxy, N-methyl amides (Weinreb amides), such as shown in Eq. 2, usually lead to formation of the ketone under similar conditions. This is particular to the Weinreb amide. Please explain. O O EtMgBr (1 equiv) OMe O OH + OMe Et Et (1) O OMe N Me EtMgBr (1 equiv) Et (2) 2 3) Predict the product for each of the following transformations and provide a mechanism. 1) nBuLi, THF, –50 °C, then (CH2O)n, –50 °C to rt 2) I2, PPh3, imidazole O O O Me C12H17O3I Mechanism: O 1) t-BuLI (2 equiv), then CO2 2) HCl 3) MeI, K2CO3 O I C8H10O3 Mechanism: 3 F O NH2 CF3 CN NaH, then HCl Me C12H9F3N2O Mechanism: 4 5) Predict the product for the following reactions. If a single enantiomer of product is expected, state so, but do not worry about which enantiomer is formed from the enantiomer of catalyst used. Pd2(dba)3 SPhos K3PO4 Cl B(OH)2 + Me Me I O SnBu3 H H Pd2(dba)3 SPhos NBn I Pd(PPh3)4 + Me ClMg Bu I + EtO2C Ph B O O Pd(PPh3)4 Pd(PPh3)4 + EtONa Br ZnBr O NC Pd(OAc)2/1 NaOtBu Cl OTf Me N + PdCl2, PPh3 Et3N C8H17 NH2 N O OAc N H Ph I CO2Et Pd(PPh3)4, NaH CO2Me CO2Me CuI (cat), K3PO4 NHMe MeHN (cat) O NaCH(CO2Me)2 cat. [Ir(COD)Cl]2 cat 3 OAc H PhCCH, 4 Et3N, Zn(OTf)2 Ph Pd2(dba)3 SPhos K3PO4 Cl B(OH)2 + O I + H Fe Me Bu 3)4 PtBu Me 2 H Me PCy2 O Pd(PPh3)4 Me SnBu3 I Pd2(dba)3 SPhos NBn ClMg PCy2 MeO 1 EtO2CPh O + B tBu CyPFJosiphos Br O OMe I ZnBr Pd(PPh 3)4 NC SPhos EtONa OTf Me N Pd(PPh3)4 + Me P Bu N O Me SnBu3 H Me Ph B O O HO+ Me O + O I H Pd(PPh3)4 Ph Br Pd(PPh3)4 NMe2 4 3 Pd(OAc)2/1 NaOtBu Cl + PdCl2, PPh3 Et3N EtONa C8H17 NH2 N O Pd(OAc)2/1 NaOtBu Cl + C8H17 NH2 N OAc N H Ph I CO2Et Pd(PPh3)4, NaH CO2Me CO2Me CuI (cat), K3PO4 NHMe (cat) MeHN O OAc CO2Me Pd(PPhOAc 3)4, NaH NaCH(CO2Me)2 cat. [Ir(COD)Cl]2 cat 3 H PhCCH, 4 Et3N, Zn(OTf)2 Ph CO2Me O PtBu2 H Fe Me PCy2 1 PhCCH, 4 Et3N, Zn(OTf)2 CyPF-tBu Josiphos PCy2 OMe MeO SPhos Me P N O Me Ph Me O 3 NMe2 HO 4 6 6) Two related reactions from the Grigg group are shown below. A) Please provide a mechanism for each reaction. B) Rationalize the observed relative stereochemistry for each transformation by providing 3-D drawings of the possible completing transition states and providing arguments for why one maybe preferred. C) Finally, as you will see from the correct mechanisms for each, the order of events differs greatly with respect to CO in each of these reactions. Please discuss what factors might influence how and when CO undergoes reaction in these types of cyclizations. O 10% Pd(OAc)2 20 mol% P(2-furyl)3 CO, NaBPh4 anisole, 120 °C O Ph 84% O I H Mechanism and 3D Drawing: TfO 10 mol% Pd(OAc)2 20 mol% PR3 Bu3N, CO morpholine 1,1-dimethylallene MeCN, 70 °C Me CO2R Me O O 60% CO2R 10% Pd(OAc)2 20 mol% P(2-furyl)3 CO, NaBPh4 anisole, 120 °C O O Ph 84% 10 mol% Pd(OAc)2 20 mol% PR3 Bu3N, CO morpholine 1,1-dimethylallene MeCN, 70 °C Me CO2R 60% O N O I TfO Me Me Me O Me H Me O N O CO2R Mechanism and 3D Drawing: 7 Me OH Me OH Me HO Me O 8. Please fill in the expected products. 9 For the following questions, please work alone. You may use Reaxys, Scifinder or Web of Knowledge for these problems. 10) Using Reaxys or Scifinder, please identify up to 3 major US commercial suppliers (ie Aldrich, Acros, Strem, etc) for each of the following. If fewer suppliers exist, please state so. (Please see note on the course Syllabus regarding commercial sources). OMe Cl Li N iPr 2·BF4 O Bu2Mg N F MeO iPr PCy2 iPr O iPr 1 11) Please identify at least one paper that provides indicated spectral data in the specified solvent. the H NMR spectra in CDCl3 for the following compounds. O PPh3 OMe 1H in CDCl3 31P in d6-acetone Please note: I have not assigned any specific problems from Grossman, Ch. 6, but you are responsible for the information in this chapter. It is a good resource for extra metal-catalyzed reaction mechanism practice. 10


![[Rh(acac)(CO)(PPh3)]: an Experimental and Theoretical Study of the](http://s3.studylib.net/store/data/007302827_1-767d92e522279b6bdb984486560992de-300x300.png)