Chemistry 652: Organometallic Chemistry - Key Spring 2013 University of Delaware
advertisement

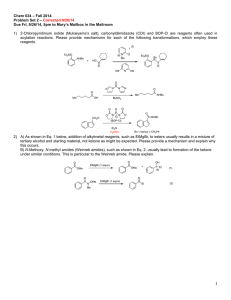
Chemistry 652: Organometallic Chemistry - Key Spring 2013 University of Delaware Prof. Donald Watson Problem Set 2 Due Beginning of Class 4/4/13 You may work in groups. No not try to fit answers on these pages, use separate paper. Do not look up answers. 1) a) PPh3 is a better sigma donor and poorer pi-acceptor than CO, so the Fe atom is more electron rich follow substitution. The appearance of the 3rd IR band indicates that the phosphines are in a cis geometry. b) Tetracyano ethylene is a very good pi-acceptor, so there is significant pibackbonding from the electron-rich Ir center. c) The para methyl group does not contribute to an increase in the cone angle as it is 180 ° from the P-Ar bond axis. The ortho group, on the other hand, is only 30° and has a significant effect. d) This indicates that the TBP complex has all of the CO equitoral (and equivalent) and the phosphines axial. PPh3 CO OC Co CO PPh3 e) Stained alkenes have a high lying homo (and are there for good sigma-donors) and a low lying lumo (and are there for good pi-acceptors). f) Alpha agnostic interaction, small JCH and attenuated C-H IR stretch. Cl Me3P H PMe3 Ta PMe3 PMe3 g) CO binds stronger to the Fe center (backbonding), does not dissociate and allow an open coordination site. H Fe PPh 3 -PPh3 Ph3P Me Me Me Fe Ph3P Me Me Fe +PPh3 H Ph3P Me – Me h) Beta-elimination possible with methyl, not with mesityl. Me Me Fe Ph3P H PPh3 Me M M M H 2. Me H Me Bridging CO IR stretch ~ 1850 cm-1 O C Cp* Rh OC Cp* Rh Rh CO Cp* 3. Me hν Si H Ph Np R(+) Mn(CO)3 Me Mn OC CO hν Si H Ph Np R(+) CO + CO OC Mn CO Me H Si Ph retention of stereochem Np MnI, d6, 18 e– tetrahedral MnIII, d4, 18 e– square pyramidal Ph (Ph3P)4Pd Br H R inversion Ph Ph3P Ph3P Pd PPh3 PPh3 Pd(0), d10, 18 e– square planar Ph Br H R H PPh3 R Pd Ph3P Br PdII, d8, 16 e– square planar + 2 PPh3 Cl (Et3P)3Pt Me CO2Et R-(+)-α-ethylchloropropionate hint: order of reactivity t-BuX > i-PrX > EtX > MeX for these types of reactions Osborne and Labinger JACS 1974 96 7145 racemization Et3P Et3P Pt PEt3 PEt3 Pt(0), d10, 18 e– square planar CO2Et H PEt3 Me Pt Et3P Cl Cl Me CO2Et + 2 PEt3 PtII, d8, 16 e– square planar R-(+)-α-ethylchloropropionate O [(Me3P)4Ir]+Cl– Ph Milstein et al. JACS 1982 104 3773 loss of stereochemistry H O Me3P Me3P Ir PMe3 PMe3 IrI, d8, 16 e– square planar Cl– O Ph Ph Me3P Ir Cl PMe3 PMe3 IrIII, d6, 18 e– square planar 4. Ph PPh3 Δ Pt Ph3P Ph Pt(0) s PPh3 Pt PPh3 Δ + Pt(PPh3)2 Pt Ph3P PPh3 Ph3P PPh3 Pt PtII, d8, 16 e– square planar Pt(0), d10, 14 e– linear Ph3P PPh3 Pt(0), d10, 18 e– square planar Ph 1,1-disubstituted (retention) This reaction is accelerated in more Lewis basic solvents. Draw a mechanism explaining why this phenomenon is observed. Lewis basic solvents accelerate trans- to cis- isomerization. Ph PPh3 Pt Ph3P PtII, d8, 16 e– square planar solv: PPh3 solv Pt PPh3 Ph solv RE PPh3 Pt Ph3P solv Pt(0), d10, 18 e– square planar Ph


![[Rh(acac)(CO)(PPh3)]: an Experimental and Theoretical Study of the](http://s3.studylib.net/store/data/007302827_1-767d92e522279b6bdb984486560992de-300x300.png)