MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering
advertisement

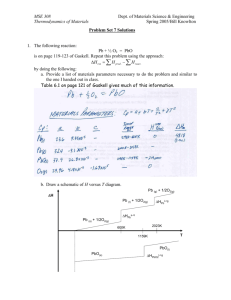
MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton Problem Set 10 Solutions 1. We derived Einstein’s famous crystal model in class. Repeat the exercise explaining all assumptions and steps. 1 of 8 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 2 of 8 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 3 of 8 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 2. For each Einstein temperature, θE, of 100K, 200K, 300K, and 500K, use a computer program other than Excel to calculate and plot the: a. partition function θE : 100 K=Blue ;200 K=Red ;300 K=Green ;500 K=Magenta 2.5 2 P 1.5 1 0.5 0 0 100 200 300 T H KL 400 500 b. Cv hv . Consider the system as a simple k cubic Einstein crystal. Of interest is the range of temperatures from 0-500K. Your plot should be similar to figure 6.1 in Gaskell. Use a mathematical program that is NOT Excel. Your plots should be labeled thoroughly. for the Einstein model as a function of T given θ E = θE : 100 K=Blue ;200 K= Red ;300 K=Green ;500 K=Magenta 25 Cv H J L mole K 20 15 10 5 0 0 100 200 T HKL 4 of 8 300 400 500 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 3. Comment on the similarities and differences between statistical thermodynamics and classical thermodynamics. Open ended problem 4. Comment on the partition function. Open ended problem 5. Derive the equation that we obtained for the Tangent method. Explain any assumptions and your steps. 5 of 8 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 6 of 8 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 7 of 8 MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 6. The change in volume of each component for a particular solution two component system are given by ∆V1 = ao X 22 (1 − 2 X 1 ) ∆V2 = 2ao X 12 X 2 Strictly using the following form of the Gibb’s-Duhem equation: 2 ∑X i =1 k d ∆ Bk = 0 prove the Gibb’s-Duhem equation is zero using the volumes given. 8 of 8