MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering
advertisement
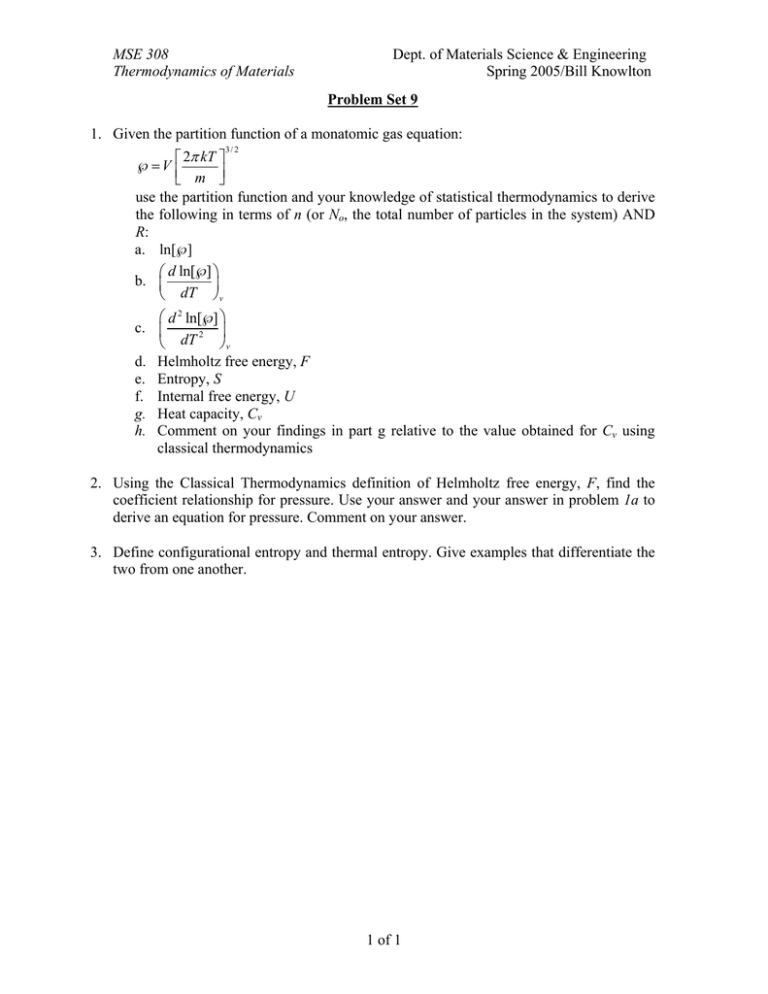
MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton Problem Set 9 1. Given the partition function of a monatomic gas equation: ⎡ 2π kT ⎤ ℘=V ⎢ ⎣ m ⎥⎦ use the partition function and your knowledge of statistical thermodynamics to derive the following in terms of n (or No, the total number of particles in the system) AND R: a. ln[℘] ⎛ d ln[℘] ⎞ b. ⎜ ⎟ ⎝ dT ⎠v 3/ 2 ⎛ d 2 ln[℘] ⎞ c. ⎜ ⎟ 2 ⎝ dT ⎠v d. Helmholtz free energy, F e. Entropy, S f. Internal free energy, U g. Heat capacity, Cv h. Comment on your findings in part g relative to the value obtained for Cv using classical thermodynamics 2. Using the Classical Thermodynamics definition of Helmholtz free energy, F, find the coefficient relationship for pressure. Use your answer and your answer in problem 1a to derive an equation for pressure. Comment on your answer. 3. Define configurational entropy and thermal entropy. Give examples that differentiate the two from one another. 1 of 1











