MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering
advertisement

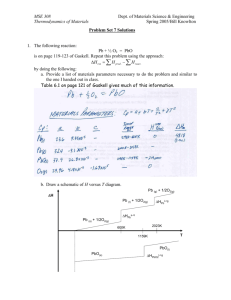
MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton Problem Set 3 Solutions 1. The Omega Potential is a Legendre transformation given by: Ω = U - µN Derive the differential equation, functionality (e.g., Ω = Ω (?,?,?)), the coefficient relations and Maxwell relations. MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 2. Derive a Legendre transformation if electrical work is done on the system where: = φ dq dw where φ is the voltage potential and q is the fundamental charge on an electron. Assume that both mechanical work and chemical work are also present. Derive the differential equation, functionality, the coefficient relations and Maxwell relations. MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 3. Extensive variables can be directly measured while intensive variables cannot be directly measured. Intensive variables need to be determined indirectly by measuring an extensive variable(s). Detail an example of determining the value for an intensive variable. A Hg thermometer is a common example of indirectly measuring an intensive variable (temperature) by directly measuring an extensive variable (volume). The system is closed, but the volume of the thermometer is larger than the volume of the system, so the volume of the Hg is allowed to increase without increasing the pressure of the system (at least to first order). Thus, we can assume that the pressure is constant. The Hg volume change is directly proportional to the Hg temperature change thus providing a means to indirectly measure the temperature of the system's surroundings. 4. Using equation 2.8 on page 24 of Gaskell, show for an ideal gas that: c p − cv = nR where R is the universal gas constant and n is the number of moles in a system. For an ideal gas, dU = 0 (p. 25), and the equation of state is given by: pV = nRT The first term on the right of equation 2.8 is just: nR ⎛ ∂V ⎞ . ⎜ ⎟ = ⎝ ∂T ⎠ p P The 2nd term in the parentheses on the right side of equation 2.8 is 0 since dU = 0. Thus, the right side of equation 2.8 is: nR c p − cv = ( P + 0) P = nR MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton 5. Using the state function of entropy as a function of T and P we developed in class, determine the change of entropy of 3 moles of lead when it is initially at 590K to 600K at 1 atm. Note that Cp(s) = 9.75x10-3 T [J/(K mole)]. See page 63- 64 Gaskell. Cp(l) = 32.4 – 3.1x10-3 T (J/K mole)) Constant pressure thus: 600 c p ∆S = ∫ dT 590 T Answer: