MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering
advertisement

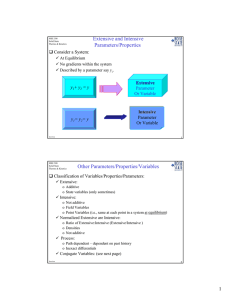
MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton Problem Set 1 1. In sections1.3, Gaskell states the final volume, V3, is independent of the order in which the steps to attain V3 are taken. Explain why this is so. Use section 1.2 to help you. 2. In section 1.6, Gaskell gives a brief synopsis of extensive and intensive properties (or variables) of a system. According to his definitions, determine whether the following properties (or variables) are extensive or intensive and explain your reasoning. a. y = y1 = y2 = y3, where y1, y2 and y3 are variables measured in a system that is in equilibrium. i. Provide several examples of variables. b. x = x1 + x2 + x3 where x1, x2 and x3 are variables measured in a system that is in equilibrium. i. Provide several examples of variables. 3. Define the following terms: a. State function b. Equation of state c. Constitution or phase diagram d. Process variables e. Homogeneous system f. Heterogeneous system g. Unary system h. Binary system i. Ternary system j. Quaternary system 4. On page 7 of Gaskell, figure 1.3a, a plot of pressure versus volume of an ideal gas is plotted at two different temperatures. a. By examining the plot, what do you think the relationship is between pressure as a function of volume? In other words, what do you think is the mathematical functionality? Explain. b. Using your reasoning from part a, use a mathematical program (not Excel) to plot figure 1.3a. Label your plot thoroughly.