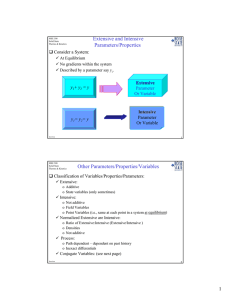
Extensive and Intensive Parameters/Properties Other Parameters/Properties/Variables Consider a System:
advertisement

Extensive and Intensive Parameters/Properties MSE 308 Thermodynamics of Materials Consider a System: 9 At Equilibrium 9 No gradients within the system 9 Described by a parameter say yi. y1 + y2 = y y1 = y2 = y Extensive Parameter Or Variable Intensive Parameter Or Variable Knowlton 1 MSE 308 Thermodynamics of Materials Other Parameters/Properties/Variables Classification of Variables/Properties/Parameters: 9 Extensive: o Additive o State variables (only sometimes) 9 Intensive: o Not additive o Field Variables o Point Variables (i.e., same at each point in a system at equilibrium) 9 Normalized Extensive are Intensive: o Ratio of Extensive:Intensive (Extensive/Intensive ) o Densities o Not additive 9 Process: o Path dependent – dependent on past history o Inexact differentials 9 Conjugate Variables: (see next page) Knowlton 2 1 Extensive and Intensive Parameters/Properties/Variables MSE 308 Thermodynamics of Materials Intensive Parameters: 9P 9T 9µ 9 ξ (electric field) 9 Any field variable 9 Ratio of Extensive/Extensive Extensive Parameters: 9Q o indeed is an extensive parameter o Process variable (not a quantity but transfers from one state to the next) 9 W (work) o indeed is an extensive parameter o Process variable (not a quantity but transfers from one state to the next) 9 9 9 9 9 9 o N/M or N/V or M/V o These are known as specific properties U (and other free energies) N V M (mass) C (heat capacity) n moles Knowlton 3 MSE 308 Thermodynamics of Materials Conjugate Variables ∑ δ W = ∑ YdX Table 2.1: Types of thermodynamic work in differential form Thermodynamic Work Knowlton Work Differential (dW) Intensive or Field Variable Extensive or State Variable thermal T•dS T = temperature S = entropy hydrostatic P•dV chemical µ•dN P = pressure µ = chemical potential V = volume N = number of particles strain – mechanical electrical σ •dε σ = stress ε = strain E•dP o E = electric field P o = polarization magnetic H•dM g H = magnetic field M g = magnetization time dependent P w•dt P w = power t = time Momentum transfer ma•dr Mass acceleration gravitational mg•dr mg = mass & gravitational acceleration & and: Voltage ⋅ dq r = distance r = distance Note: The intensive and extensive variable pairs are known as conjugate variables. For example, T and S are conjugate variables. 4 2 MSE 308 Thermodynamics of Materials Equilibrium Some Thermodynamic Potential, E Types of Equilibrium States: 9 Stable 9 Metastable 9 Unstable 10 8 Transition State 6 Unstable Equilibrium State 4 2 0 Metastable Equilibrium State -2 -4 -6 Stable Equilibrium State -8 -2 Knowlton -1 0 1 Reaction Coordinates, ξ 2 Knowlton 5 MSE 308 Thermodynamics of Materials Comments on Energy, Entropy, Heat & Work Energy and Entropy: 9 "It would be preferable, but for the need for economy of words, to speak always of 'energy function' and 'entropy function' rather than of energy and entropy. 9 They are not material entities, but are mathematical functions having certain properties. Versus Heat and Work: 9 It is always permissible to speak of the energy and entropy content of a body relative to some other State. 9 In the same light, it is not permissible to speak of a body's heat or work content. 9 Heat and work are modes of transfer of energy between one body and another. Hence, they are path dependent and process variables. Knowlton » Bridgman, The Nature of Thermodynamics (Harvard, 1941) » Denbigh, The Principles of Chemical Equilibrium (Cambridge University Press, 1981) 6 3