Thermodynamics: Exact Differentials & First Law
advertisement
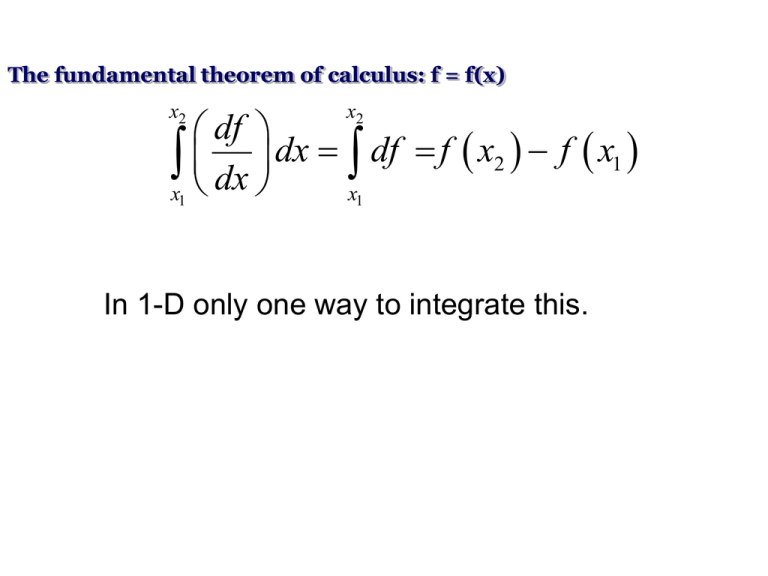
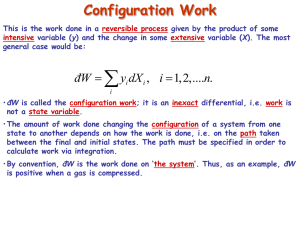
The fundamental theorem of calculus: f = f(x) x2 x2 df x dx dx x df f x2 f x1 1 1 In 1-D only one way to integrate this. In 2-D infinite paths possible y z = z(x,y) A C a b x For an exact differential b a b b a b a a dz dz dz dz dz 0 C Green’s theorem y A C a b x N M Mdx Ndy dxdy C A x y In general, the differential dz = Mdx + Ndy is ‘exact’ if: M N 1. y x 2. dz 0 C b 3. dz Is path independent a e.g. state variables, P = P(V,T) Inexact differentials do not satisfy these conditions e.g. dz = ydx - xdy Sum of two inexact differentials can be exact. Heat: Spontaneous flow of energy caused by the temperature difference between two objects Work: Any other kind of energy transfer Internal Energy: Total energy in a system First law of thermodynamics (in words) Increase in internal energy = Heat added + work done on the system U = Q + W (W is the work done ON the system) First law of thermodynamics (in words) Increase in internal energy = Heat added + work done on the system U = Q + W (W is the work done ON the system) Phlogiston? First law of thermodynamics dQ = dU - dW Show PdV Configuration Work This is the work done in a reversible process given by the product of some intensive variable (y) and the change in some extensive variable (X). The most general case would be: đW y dX , i i i 1,2,....n. i •đW is called the configuration work; it is an inexact differential, i.e. work is not a state variable. •The amount of work done changing the configuration of a system from one state to another depends on how the work is done, i.e. on the path taken between the final and initial states. The path must be specified in order to calculate work via integration. What do we mean by reversible? Why is it an inexact differential? Configuration Work This is the work done in a reversible process given by the product of some intensive variable (y) and the change in some extensive variable (X). The most general case would be: đW y dX , i i i i 1,2,....n. Dissipative Work • This is the work done in an irreversible process; it is always done ‘on the system’. • Total work is the algebraic sum any configuration work and any dissipative work. • If a process is reversible, then dissipation is necessarily zero. Examples: Stirring Resistive electrical heating Frictional work Plastic deformation Many chemical reactions Microwave oven Do not confuse between exact differentials and reversible processes. Reversible processes need not be exact differentials. Why is it called reversible then?