Aryl Diazonium Salts. New Coupling Agents and Surface Science Brochure
advertisement

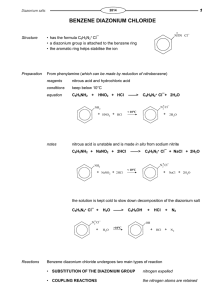
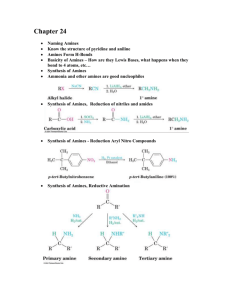
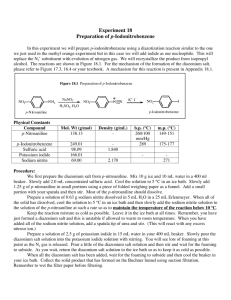
Brochure More information from http://www.researchandmarkets.com/reports/2180116/ Aryl Diazonium Salts. New Coupling Agents and Surface Science Description: Diazonium compounds are employed as a new class of coupling agents to link polymers, biomacromolecules, and other species (e. g. metallic nanoparticles) to the surface of materials. The resulting high performance materials show improved chemical and physical properties and find widespread applications. The advantage of aryl diazonium salts compared to other surface modifiers lies in their ease of preparation, rapid (electro)reduction, large choice of reactive functional groups, and strong aryl–surface covalent bonding. This unique book summarizes the current knowledge of the surface and interface chemistry of aryl diazonium salts. It covers fundamental aspects of diazonium chemistry together with theoretical calculations of surface–molecule bonding, analytical methods used for the characterization of aryl layers, as well as important applications in the field of electrochemistry, nanotechnology, biosensors, polymer coatings and materials science. Furthermore, information on other surface modifiers (amines, silanes, hydrazines, iodonium salts) is included. This collection of 14 self–contained chapters constitutes a valuable book for PhD students, academics and industrial researchers working on this hot topic. Contents: Preface XV List of Contributors XVII 1.6.1 Chemical Structure 21 1.6.2 The Spatial Structure of the Layers 22 1.6.3 Compactness of the Layers 23 1.6.4 Swelling of the Layer 24 1.6.5 Electron Transfer through the Layers 24 1.6.6 The Formation Mechanism of Multilayers 25 1.7 Conclusion 27 References 27 2 Aryl Surface Bonding: A Density Functional Theory (DFT) Simulation Approach 37 Nan Shao, Sheng Dai, and De–en Jiang 2.1 Introduction 37 2.2 Density Functional Theory 38 2.3 Bonding between Aryl and Various Substrates 38 2.3.1 On Graphite/Graphene 39 2.3.1.1 On the Basal Plane 39 2.3.1.2 On the Edges of Graphene 42 2.3.2 On Carbon Nanotubes 44 2.3.3 On Metal Surfaces 45 2.4 Summary and Outlook 48 Acknowledgments 49 References 50 3 Patterned Molecular Layers on Surfaces 53 Alison J. Downard, Andrew J. Gross, and Bradley M. Simons 3.1 Methods Based on Scanning Probe Lithography 53 3.1.1 AFM 54 3.1.2 SECM 54 3.1.3 Spotting 56 3.2 Methods Based on Soft Lithography 57 3.2.1 Printing 57 3.2.2 Molds 59 3.2.3 Nanosphere Lithography 59 3.3 Methods Based on Lithography 60 3.4 Methods Based on Surface–Directed Patterning 62 3.4.1 Modifi cation of Si Surfaces 63 3.4.2 Modifi ed Electrode Arrays 64 3.5 Summary and Conclusions 66 References 68 4 Analytical Methods for the Characterization of Aryl Layers 71 Karsten Hinrichs, Katy Roodenko, Jörg Rappich, Mohamed M. Chehimi, and Jean Pinson 4.1 Introduction 71 4.2 Scanning Probe Microscopies 71 4.3 UV VIS Spectroscopy: Transmission, Reflection, and Ellipsometry 72 4.4 IR Spectroscopy 72 4.4.1 Transmission Spectroscopy 73 4.4.2 Refl ection Spectroscopy 74 4.4.3 Infrared Spectroscopic Ellipsometry (IRSE) 75 4.4.4 IRSE Surface Characterization 77 4.4.5 In Situ IR Spectroscopy: ATR and IRSE 79 4.5 Raman Spectroscopy and Surface–Enhanced Raman Scattering (SERS) 83 4.6 X–ray Photoelectron Spectroscopy (XPS) 84 4.7 X–ray Standing Waves (XSW) 91 4.8 Rutherford Backscattering 93 4.9 Time of Flight Secondary Ion Mass Spectroscopy 93 4.10 Electrochemistry 94 4.11 Contact Angle Measurements 96 4.12 Conclusion 96 References 98 5 Modification of Nano–objects by Aryl Diazonium Salts 103 Dao–Jun Guo and Fakhradin Mirkhalaf 5.1 Introduction 103 5.2 Electrochemical Modifi cation of Nano–objects by Reduction of Diazonium Salts 105 5.2.1 Surface Modifi cation of Carbon Nano–objects via Electrochemical Reduction of Aryl Diazonium Cations 105 5.2.2 Surface Modifi cation of Metal and Metal Oxide Nano–objects via Electrochemical Reduction of Aryl Diazonium Cations 111 5.3 Chemical Modification of Nano–objects by Reduction of Diazonium Salts 112 5.3.1 Surface Modifi cation of Carbon Nano–objects via Chemical Reduction of Aryl Diazonium Cations 112 5.3.2 Surface Modifi cation of Metal and Metal Oxide Nano–objects via Chemical Reduction of Aryl Diazonium Cations 116 5.4 Summary and Conclusions 119 Acknowledgments 120 References 120 Methods and Applications 125 Sarra Gam–Derouich, Samia Mahouche–Chergui, Hatem Ben Romdhane, and Mohamed M. Chehimi 6.1 Introduction 125 6.2 Methods for Grafting Coupling Agents from Aryl Diazonium Compounds 127 6.3 Grafting Macromolecules to Surfaces through Aryl Layers 130 6.3.1 Binding Macromolecules to Surfaces by a Grafting from Strategy 130 6.3.1.1 Surface–Initiated Atom Transfer Radical Polymerization (SI–ATRP) 130 6.3.1.2 Surface–Initiated Reversible Addition Fragmentation Chain Transfer (SI–RAFT) 142 6.3.1.3 Surface–Initiated Photopolymerization 143 6.3.1.4 Alternative Methods 146 6.3.2 Attachment of Macromolecules through Grafting onto Strategies 147 6.3.2.1 Photochemical Attachment 147 6.3.2.2 Ring Opening 148 6.3.2.3 Acylation 149 6.3.2.4 Click Chemistry 149 6.3.2.5 Diazotation of Substrates and Macromolecules 150 6.4 Adhesion of Polymers to Surfaces through Aryl Layers 151 6.5 Conclusion 153 References 153 7 Grafting Polymer Films onto Material Surfaces: The One–Step Redox Processes 159 Guy Deniau, Serge Palacin, Alice Mesnage, and Lorraine Tessier 7.1 Cathodic Electrografting (CE) in an Organic Medium 160 7.1.1 Direct Cathodic Electrografting of Vinylic Polymers 160 7.1.2 Indirect Cathodic Electrografting 162 7.2 Surface Electroinitiated Emulsion Polymerization (SEEP) 164 7.2.1 Characterization of Poly(Butyl Methacrylate) Films 166 7.2.2 Determination of the Film Structure 167 7.2.3 Reduction of Protons and the Role of Hydrogen Radicals 169 7.2.4 Mechanism of SEEP 170 7.3 Chemical Grafting via Chemical Redox Activation (Graftfast ) 171 7.3.1 Process without Vinylic Monomer 172 7.3.2 Process with Vinylic Monomer 174 7.3.2.1 Type of Materials 174 7.3.2.2 Parameters Controlled in the Process 174 7.4 Summary and Conclusions 177 References 178 8 Electrografting of Conductive Oligomers and Polymers 181 Jean Christophe Lacroix, Jalal Ghilane, Luis Santos, Gaelle Trippe–Allard, Pascal Martin, and Hyacinthe Randriamahazaka 8.1 Introduction 181 8.2 Conjugated Oligomers and Polymers 181 8.3 Surface Grafting Based on Electroreduction of Diazonium Salts 184 8.4 Polyphenylene and Oligophenylene–Tethered Surface Prepared by the Diazonium Reduction of Aniline or 4–Substituted Aniline 187 8.5 n–Doping and Conductance Switching of Grafted Biphenyl, Terphenyl, Nitro–biphenyl and 4–Nitroazobenzene Mono– and Multilayers 187 8.6 p–Doping and Conductance Switching of Grafted Oligo–Phenylthiophene or Oligothiophene Mono– and Multilayers 190 8.7 p–Doping and Conductance Switching of Grafted Oligoaniline Mono– and Multilayers 192 8.8 Conclusion and Outlook 193 References 195 9 The Use of Aryl Diazonium Salts in the Fabrication of Biosensors and Chemical Sensors 197 J. Justin Gooding, Guozhen Liu, and Alicia L. Gui 9.1 Introduction 197 9.1.1 Sensors and Interfacial Design 197 9.1.2 Molecular Level Control over the Fabrication of Sensing Interfaces 198 9.2 The Important Features of Aryl Diazonium Salts with Regard to Sensing 200 9.3 Sensors and Biosensors Fabricated Using Aryl Diazonium Salts 201 9.3.1 Chemical Sensors Sensors Fabricated via the Immobilization of Chemical Recognition Species 201 9.3.2 Biosensors 205 9.3.2.1 Enzyme Biosensors 206 9.3.2.2 Immunobiosensors 208 9.3.2.3 DNA–Based Biosensors 210 9.3.2.4 Cell–Based Biosensors 213 9.4 Conclusions 213 References 214 10 Diazonium Compounds in Molecular Electronics 219 Richard McCreery and Adam Johan Bergren 10.1 Introduction 219 10.2 Fabrication of Molecular Junctions Using Diazonium Reagents 222 10.2.1 Substrates for Diazonium–Derived Molecular Junctions 222 10.2.2 Surface Modifi cation Using Diazonium Chemistry 223 10.2.3 Application of Top Contacts 225 10.3 Electronic Performance of Diazonium–Derived Molecular Junctions 226 10.3.1 Surface Diffusion Mediated Deposition (SDMD) 227 10.3.2 Structural Control of Molecular Junction Behavior 230 10.3.3 Redox Reactions in Molecular Junctions 232 10.3.4 Microfabricated Molecular Devices Made with Diazonium Chemistry 233 10.4 Summary and Outlook 235 Acknowledgments 236 References 236 11 Electronic Properties of Si Surfaces Modified by Aryl Diazonium Compounds 241 Jörg Rappich, Xin Zhang, and Karsten Hinrichs 11.1 Introduction 241 11.2 Experimental Techniques to Characterize Electronic Properties of Si Surfaces in Solutions 242 11.2.1 In Situ Photoluminescence and Photo Voltage Measurements 242 11.2.2 In Situ PL and PV Measurements during Electrochemical Grafting 244 11.2.3 Reaction Scheme of the Electrochemical Grafting via Diazonium Ions 245 11.2.4 Change in IPL and UPV during Electrochemical Grafting onto Si Surfaces 246 11.2.5 Change in Band Bending and Work Function after Electrochemical Grafting onto Si Surfaces 248 11.2.6 pH Dependence and Enhanced Surface Passivation 249 11.3 Conclusion and Outlook 251 Acknowledgments 252 References 252 12 Non–Diazonium Organic and Organometallic Coupling Agents for Surface Modification 255 Fetah I. Podvorica 12.1 Amines 255 12.1.1 Characterization of the Grafted Layer 257 12.1.1.1 Electrochemical Methods 257 12.1.1.2 Surface Analysis Techniques 258 12.1.2 Chemical Grafting 259 12.1.3 Localized Electrografting 260 12.1.4 Grafting Mechanism 261 12.1.5 Applications 262 12.2 Arylhydrazines 264 12.3 Aryltriazenes 266 12.4 Alcohols 267 12.4.1 Observation and Characterization of the Film 268 12.4.2 Applications 269 12.5 Grignard Reagents 270 12.5.1 Characterization of the Layers 271 12.5.2 Grafting Mechanism 272 12.6 Onium Salts 272 12.6.1 Iodonium Salts 272 12.6.2 Sulfonium Salts 273 12.6.3 Ammonium Salts 273 12.7 Alkyl Halides 274 12.8 Conclusion 275 References 276 13 Various Electrochemical Strategies for Grafting Electronic Functional Molecules to Silicon 283 Dinesh K. Aswal, Shankar Prasad Koiry, and Shiv Kumar Gupta 13.1 Introduction 283 13.2 Architecture of Hybrid Devices 284 13.2.1 Molecular Dielectrics and Wires 285 13.2.2 Molecular Diodes 286 13.2.3 Resonant Tunnel Diodes 286 13.2.4 Molecular Transistors 286 13.3 Electrografting of Monolayers to Si 287 13.3.1 Essential Requirements 287 13.3.2 Experimental Process of Electrografting 287 13.4 Negative Differential Resistance Effect in a Monolayer Electrografted Using a Diazonium Complex 288 13.4.1 Electrografting of DHTT 288 13.4.2 NDR Effect in DHTT Monolayers 290 13.5 Dielectric Monolayers Electrografted Using Silanes 293 13.5.1 Mechanism of Electrografting 293 13.5.2 Electrical Characterization 294 13.6 Molecular Diodes Based on C60/Porphyrin–Derivative Bilayers 295 13.6.1 Fabrication Process 296 13.6.1.1 Electrografting of Acceptor C60 Layer on Si 296 13.6.1.2 Self–Assembly of Donor Porphyrin Derivative Layer on C60/Si 297 13.6.2 Rectifi cation Characteristics of D A Bilayers 298 13.7 Memory Effect in TPP–C11 Monolayers Electrografted Using a C=C Linker 301 13.7.1 Electrografting of TPP–C11 Monolayer 301 13.7.2 Electrical Bistability and Memory Effect 303 13.8 Summary 305 References 305 14 Patents and Industrial Applications of Aryl Diazonium Salts and Other Coupling Agents 309 James A. Belmont, Christophe Bureau, Mohamed M. Chehimi, Sarra Gam–Derouich, and Jean Pinson 14.1 Introduction 309 14.2 Patents 309 14.2.1 The Surface Chemistry of Diazonium Salts 309 14.2.2 The Surface Chemistry of Other Coupling Agents 310 14.2.3 Post–Modifi cation of the Grafted Layers 310 14.2.4 Composite Materials 310 14.2.5 The Surface Modifi cation of Nano–objects 312 14.2.6 Microelectronics 312 14.2.7 Biomedical Applications 312 14.2.8 Sensors, Biosensors, Surfaces for Biological Applications 312 14.2.9 Energy Conversion 313 14.3 Industrial Applications 313 14.3.1 The Development of Modifi ed Carbon Blacks 313 14.3.2 Industrial Applications of the Electropolymerization of Vinylics: Alchimer and AlchiMedics 314 14.3.2.1 From Research to Development 314 14.3.2.2 Application of eG to Drug–Eluting Stents: AlchiMedics 315 14.3.2.3 Application of eG to Copper Interconnects: Alchimer 317 14.4 Conclusion 319 References 319 Index Ordering: Order Online - http://www.researchandmarkets.com/reports/2180116/ Order by Fax - using the form below Order by Post - print the order form below and send to Research and Markets, Guinness Centre, Taylors Lane, Dublin 8, Ireland. Page 1 of 2 Fax Order Form To place an order via fax simply print this form, fill in the information below and fax the completed form to 646-607-1907 (from USA) or +353-1-481-1716 (from Rest of World). If you have any questions please visit http://www.researchandmarkets.com/contact/ Order Information Please verify that the product information is correct. Product Name: Aryl Diazonium Salts. New Coupling Agents and Surface Science Web Address: http://www.researchandmarkets.com/reports/2180116/ Office Code: SCVU6PEV Product Format Please select the product format and quantity you require: Quantity Hard Copy (Hard Back): USD 188 + USD 29 Shipping/Handling * Shipping/Handling is only charged once per order. Contact Information Please enter all the information below in BLOCK CAPITALS Title: First Name: Mr Mrs Dr Miss Last Name: Email Address: * Job Title: Organisation: Address: City: Postal / Zip Code: Country: Phone Number: Fax Number: * Please refrain from using free email accounts when ordering (e.g. Yahoo, Hotmail, AOL) Ms Prof Page 2 of 2 Payment Information Please indicate the payment method you would like to use by selecting the appropriate box. Pay by credit card: You will receive an email with a link to a secure webpage to enter your credit card details. Pay by check: Please post the check, accompanied by this form, to: Research and Markets, Guinness Center, Taylors Lane, Dublin 8, Ireland. Pay by wire transfer: Please transfer funds to: Account number 833 130 83 Sort code 98-53-30 Swift code ULSBIE2D IBAN number IE78ULSB98533083313083 Bank Address Ulster Bank, 27-35 Main Street, Blackrock, Co. Dublin, Ireland. If you have a Marketing Code please enter it below: Marketing Code: Please note that by ordering from Research and Markets you are agreeing to our Terms and Conditions at http://www.researchandmarkets.com/info/terms.asp Please fax this form to: (646) 607-1907 or (646) 964-6609 - From USA +353-1-481-1716 or +353-1-653-1571 - From Rest of World