A Parallel Microfluidic Channel Fixture Fabricated
advertisement

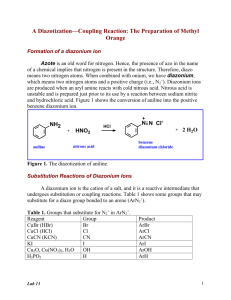
A parallel microfluidic channel fixture fabricated using laser ablated plastic laminates for electrochemical and chemiluminescent biodetection of DNA Thayne L. Edwards, Jason C. Harper, Ronen Polsky, DeAnna M. Lopez, David R. Wheeler, Amy C. Allen, and Susan M. Brozik* Biosensors & Nanomaterials, Sandia National Laboratories, PO Box 5800, MS-0892, Albuquerque, NM 87185 USA Telephone: (505) 844-5105; Fax: (505) 845-8161; Email:smbrozi@sandia.gov Supplemental Information Electrografting of Aryl Diazonium Salts in Ti/Au Fixture Figures S1-S6 Electrochemical DNA Detection Assay 1 Electrografting of Aryl Diazonium Salts in Ti/Au Fixture Selective functionalization of individual electrodes in the microfluidic fixture was realized via bias-assisted grafting of aryl diazonium salts. The electrochemical reduction of an aryl diazonium salt creates an aryl centered radical with elimination of dinitrogen.1 These aryl radicals can then react with conducting or semiconducting surfaces forming a covalent bond. While this is a simplified description of the grafting, and the nature of this bond with Au substrates is still under investigation, the resulting thin film is highly stable, withstanding several minutes of ultrasonication and long-term storage in air while remaining conductive, which is critical for subsequent electrochemical measurements.2 Bias-assisted deposition of carboxylphenyl and nitrophenyl diazonium onto the thermally evaporated Ti/Au working electrodes (WEs) in the fixture (using the common on-chip Au pseudo-reference and Pt counter electrodes) was studied using voltammetry. Cyclic voltammograms of the respective diazonium salts are presented in Figure S1. A sharp irreversible peak and a broad cathodic wave was observed on the first scan with carboxylphenyl diazonium at Ep,c = -180 mV and -540 mV, respectively, vs. the Au pseudo-reference (see Figure S1A, black trace), and are attributed to the reduction of the diazonium salt. Multiple reduction peaks for diazonium reduction have been observed on Au electrodes and have been attributed to reduction through different crystallographic phases on the Au surface.3 Both peaks were significantly suppressed and negatively shifted by ~100 mV on the second CV scan (Figure S1A, grey trace). The higher overpotential required for reaction and lower relative currents on the second scan are characteristic of electrochemistry at films derived from diazonium electrodeposition, and are a manifestation of the higher resistance to electron transfer through the phenyl thin film. Nitrophenyl diazonium electrodeposition yielded two sharp irreversible peaks at Ep,c = -160 mV and -410 mV (Figure S1B, black trace). No Faradic currents were observed on the subsequent CV scan (Figure S1 B, grey trace) and the overall current response was significantly suppressed, again characteristic of thin film formation via bias-assisted grafting. The electrodeposition protocol used to form the grafted thin film has been shown to have a profound affect on the order, thickness, and electron transfer kinetics of the resulting film.4 Previous results have shown that fix potential depositions lead to thinner 2 and more ordered films, allowing for improved current responses, than potential sweep methods which produce thicker films that can hinder electron transfer.4 Therefore, chronoamperometric electrodeposition was used to modify WEs with thin films to immobilize ssDNA probes (see Experimental Methods section for parameters). Figure S1, C and D show the current responses with time for carboxylphenyl diazonium deposition at 6 Au WEs, and nitrophenyl diazonium deposition at 3 Au WEs, respectively, in a 9 WE microfluidic fixture. An initial period of high cathodic current is observed corresponding to diazonium reduction and double layer charging. The current response then decreases as the thin film forms and resistance to electron transfer increases. A low degree of variability in current response during electrodeposition between all electrodes on the array strongly suggests consistent deposition of the diazonium salt across all electrodes. REFERENCES 1. (a) M. Delamar, R. Hitmi, J. Pinson, and J.-M. Savéant, J. Am. Chem. Soc. 1992, 114, 5883–5884. (b) M.-C. Bernard, A. Chaussé, E. Cabet-Deliry, M. M. Chehimi, J. Pinson, F. Podvorica, and C. Vautrin-Ul, Chem. Mater. 2003, 15, 3450-3462. 2. (a) G. Z. Liu, T. Böcking, and J. J. Gooding, J. Electroanal. Chem., 2007, 600, 335344. (b) A. Laforgue, T. Addou, and D. Bélanger, Langmuir 2005, 21, 6855-6865. (c) J. C. Harper, R. Polsky, D. R. Wheeler, and S. M. Brozik, Langmuir 2008, 24, 22062211. 3. A. Benedetto, M. Balog, P. Viel, F. Le Derf, M. Sallé, and S. Palacin, Electrochim. Acta, 2008, 53, 7117-7122. 4. (a) J. C. Harper, R. Polsky, S. M. Dirk, D. R. Wheeler, and S. M. Brozik, Electroanalysis 2007, 19, 1268-1274. (b) H. Uetsuka, D. Shin, N. Tokuda, K. Saeki, and C. E. Nebel, Langmuir 2007, 23, 3466-3472. (c) J. C. Harper, R. Polsky, D. R. Wheeler, S. M. Dirk, and S. M. Brozik, Langmuir, 2007, 23, 8285-8287. (d) J. Haccoun, C. Vautrin-Ul, A. Chaussé, and A. Adenier, Prog. Org. Coat. 2008, 63, 1824. (e) J. C. Harper, R. Polsky, D. R. Wheeler, D. M. Lopez, D. C. Arango, and S. M. Brozik, Langmuir, 2009, 25, 3282-3288. 3 Figure S1 Figure S1. Cyclic voltammograms of A) 1 mM carboxylphenyl diazonium and B) 1 mM nitrophenyl diazonium at unmodified 500 M diameter Au WEs with on-chip Au pseudo-reference and Pt counter electrodes in 0.1 M Bu4NBF4, in ACN, v = 100 mV s-1. Initial (black) and second (grey) CV scans are shown. Chronoamperometric electrodeposition of C) carboxylphenyl diazonium at 6 of the 9 Au WEs and D) nitrophenyl diazonium at 3 of the 9 Au WEs on the microfluidic electrode array. 1mM diazonium salt in 0.1 M Bu4NBF4, in ACN, applied potential = -1.0 V vs. Au pseudoreference. 4 Figure S2 Figure S2. Cyclic voltammograms of 1 mM Fe(CN)63-/4- in 0.1 M KCl, v = 100 mV∙s-1, at each of nine individually addressable 500 m diameter Au electrodes vs. on-chip common Au pseudo-reference, and shared Pt counter. The second CV scan is shown. 5 Figure S3 Figure S3. Cyclic voltammograms of 1 mM Fe(CN)63-/4- in 0.1 M KCl, v = 100 mV∙s-1, at each of nine individually addressable 500 m diameter ITO electrodes vs. on-chip common Au pseudo-reference, and shared Pt counter. The second CV scan is shown. 6 Figure S4 Figure S4. Cyclic voltammogram of 5 mM ruthenium bipyridine, 50 mM tripropylamine in 1x PBS, pH 7.4, at an ITO working electrode in a plastic laminate fixture. On-chip Au pseudo-reference and Pt counter electrodes; v = 100 mV/s. Figure S5 Figure S5. Plot of electrochemiluminecent reaction light intensity imaged through the underside of an ITO electrode vs. applied potential during a cyclic voltammetry sweep from 0 to 2 to 0 volts vs. the on-chip Au pseudo-reference electrode. 5 mM ruthenium bipyridine, 50 mM tripropylamine in 1x PBS, pH 7.4. On-chip Pt counter; v = 100 mV/s. 7 Figure S6 Figure S6. Electrochemical DNA Detection Assay Electrochemical DNA Detection Assay Array channels were treated with 10 M of ssDNA or dsDNA sequences 2 (breast cancer target), 5 (colorectal cancer target), and/or 7 (random control) in 2x SSC for 2 hours at room temperature. Following the 2 hour treatment with analyte solution, the 8 channel was thoroughly rinsed with 2× SSC, resulting in a surface with bound target DNA (Figure S6, step 1). This surface was then treated with 10 M of biotinlyated DNA detection sequence 3 and 6 together (detection probes) in 2× SSC solution for 2 hours at room temperature. These detection sequences will bind to the surface only if target DNA sequences were present in step 1 and bound to the electrode surface. After treatment, the array was rinsed twice with 2× SSC solution (Figure S6, step 2). The array channels were then incubated with a 1:1000 dilution of ExtrAvidin-HRP (electrochemical label, HRP = horseradish peroxidase) in peroxidase stabilizer solution for 45 minutes. Afterwards, the channels were rinsed with 1× PBS, pH 7.4 (Figure S6, step 3). For detection, a given channel was exposed to TMB conductivity solution, which contains HRP substrate (H2O2) and TMB mediator (Figure S6, step 4). The chronoamperometric current at each working electrode was measured at -300 mV vs. the common Au pseudo-reference. The current measured is a function of bound HRP catalysis, with TMB serving as an electron mediator between the Au electrode and the heme cofactor of the redox active enzyme. 9