Alkyl Halides 10.1 Naming alkyl halides- Read 10.2 Structure of alkyl halides
advertisement
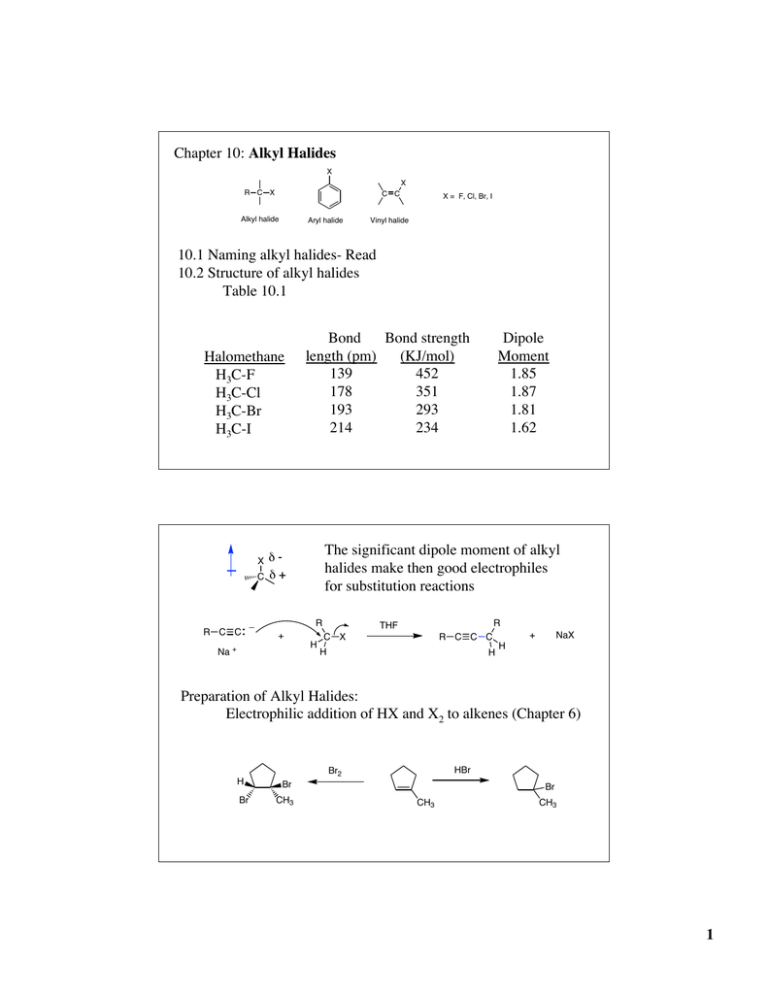
Chapter 10: Alkyl Halides X X R C X C C Alkyl halide Aryl halide X = F, Cl, Br, I Vinyl halide 10.1 Naming alkyl halides- Read 10.2 Structure of alkyl halides Table 10.1 Halomethane H3C-F H3C-Cl H3C-Br H3C-I X C R C C Bond Bond strength length (pm) (KJ/mol) 139 452 178 351 193 293 214 234 The significant dipole moment of alkyl halides make then good electrophiles for substitution reactions dd+ R _ + Na + Dipole Moment 1.85 1.87 1.81 1.62 H R THF C X R C C C H H + NaX H Preparation of Alkyl Halides: Electrophilic addition of HX and X2 to alkenes (Chapter 6) HBr Br2 H Br Br CH3 Br CH3 CH3 1 Free radical halogenation Mechanism of free radical halogenation (Chapter 5) • three distinct steps 1. Initiation 2. Propagation 3. Termination Free radical chlorination is not very useful for making alkyl chlorides polychlorination non-specific chlorination H H H H H H H H Cl2, hn H H Cl H H C C C C H H C C C C Cl H H H H H H H H H H H H (30%) (70%) four 2° hydrogens six 1° hydrogens H H C H H H Cl2, hn + H C C C C H H H H C H H H H C H H H + H C C C H H C C C Cl H H H H C C C H H H H H Cl H one 3° hydrogens nine 1° hydrogens (35%) (65%) Relative reactivity of hydrogens toward free radical chlorination H H R R C H R C H R C H H Primary (1°) hydrogens R < Secondary (2°) hydrogens R < 3.5 1.0 Tertiary (3°) hydrogens 5.0 Reactivity is reflective of radical stability H H R C• H Primary (1°) R R C• R C• R < Secondary (2°) R < Tertiary (3°) H H R R C H R C H R C H H DH° = 420 KJ/mol R R 401 KJ/mol 390 KJ/mol 2 Free radical bromination is much more selective for the most stable radical intermediate. H H H C H H H H H C H H H H C H H H X2, hn H C C C H H H H + H C C C X H C C C H H H H H X H X = Cl X = Br 65 : 35 1 : 99 The propagation step for free radical bromination is endergonic, as opposed to chlorination which is exergonic. According to the Hammond postulate the transition state for bromination should resemble the product radical, and therefore be more selective for the product going through the more stable radical intermediate R3C-H + X• X = Cl X = Br R3C• + HX DH° = -50 KJ/mol DH° = +12 KJ/mol 3 Allylic Bromination of Alkenes allylic position is the next to a double bond allylic hydrogen C C C H allylic carbon Br NBS, hn CCl4 Allylic bromination of an alkene takes place through a free radical mechanism. Radical Stability H C C• H H C• H Vinylic < < Primary (1°) R < C R C• R C• H Methyl • R R C• R < Secondary (2°) Tertiary (3°) < Benzylic ~ _ C C• Allylic Increasing stability C C DH° (KJ/mol) = 444 H H H H R H C H R C H R C H R C H H H R R 438 420 401 390 C C 361 C H H 368 Radicals are also stablized by hyperconjugation 4 Allylic radical is resonance stabilized Recall (and please review) from Chapter 2: resonance forms- atoms remain fixed in all resonance forms. Resonance forms differ only by the placement of electrons No one resonance form is entirely accurate. The actual structure is a hybrid of all the resonance forms. Resonance forms do not necessarily contribute equally to the resonance hybrid. The greater the number of resonance structures the more stable the resonance hybrid. C C • C • C 1/2 • C C C C 1/2 • C 1 1/2 bonds Map of the electron density due to the unpaired electron (the “spin”) 5 NBS, hn R R R • • R R + Br Br + Br (17 %) R (83 %) Br KOH NBS, hn 1,3-cyclohexadiene (conjugate diene) Alkyl halides from alcohols the most general method of preparing alkyl halides 1. Substitution reaction of alcohols with HX R-OH + HX R-X + H2O Works better with more substituted alcohols H H C OH H > R C OH H H Methyl Primary (1°) H > R C OH R R > R C OH R Secondary (2°) Tertiary (3°) increasing reactivity Polar mechanism with a carbocation intermediate. Reactivity reflects the stability of the carbocation intermediate (Chapter 11). One drawback to this method is that carbocations can rearrange. 6 2. Prepartion of alkyl chlorides by the treatment of alcohols with thionyl chloride (SOCl2) R-OH + SOCl2 R-Cl + SO2 + HCl 3. Prepartion of alkyl bromides by the treatment of alcohols with Phosphorous tribromide (PBr3) R-OH + PBr3 R-Br + P(OH)3 These methods work best on primary and secondary alcohols. They do not work at all for tertiary alcohols . . . more reactions of alkyl halides: Grignard reagents Alkyl halides will react with some metals (M0) in ether or THF to form organometallic reagents Grignard reagent- organomagnesium R-X + ether or THF Mg(0) R-Mg(II)-X X= Cl, Br, I R can be a variety of organic groups: 1°-, 2°-, 3°-alkyl, aryl or vinyl d- d+ C MgX _ C carbon nuccleophile (recall acetylide anion) Carbanions: nucleophile react with electrophile 7 R-X H2O R-MgX R-H Grignard reagents are most commonly used in reactions with carbonyl compounds (Chapter 19). Lab: _ MgBr Br Mg(0) ether _ O O O O _ H3O C OH + O Organometallic coupling reactions: organolithium reagents R-X Li(0) R-Li + pentane d- d+ _ C C Li LiX very strong bases very strong nucleophiles organolithium reagents are most commonly used as very strong bases and in reactions with carbonyl compounds Cuprates (Gilman’s reagent) ether 2 CH3Li + H3C CuI _ Cu Li+ + LiI H3C Gilman's reagent (dimethylcuprate, dimethylcopper lithium) 8 - R2CuLi = R strong nucleophiles Nucleophilic substitution reaction with alkyl halides (alkylation) CH3(CH2)8CH2-I + (CH3)2CuLi ether CH3(CH2)8CH2-CH3 + CH3Cu + LiI Reaction with vinyl and aryl halides H H (CH3)2CuLi I CH3 H H double bond geometry is preserved Br (CH3)2CuLi CH3 Oxidation [O]: the loss of electrons. Decrease in electron density on carbon by forming a C-O, C-N, C-X bonds or by breaking C-H bonds Increase in O, N, X content or decrease in H content Reduction [H]: the gain of electrons Increase in electron density on carbin by forming C-H bonds or breaking C-O, C-N, C-X bonds increase on H content or decrease in O, N, X content 9 CH4 + Cl2 H3C-Cl H Mg H3O+ oxidation CH4 reduction + Br2 H H H H H H H H H C C H H H H C C C C H H H C C H H H C C H H H H H OH H + H2O Reduction H Br H + HBr Oxidation H H H Pd + H2 C C Br Br H C C H 1) 2) H3C-Cl C C H H H Neither [O] or [H] Neither [O] or [H] (hydration) Table 10.5: Increasing oxidation state C C C C C C O C OH C NH2 C O C NH C CO2 OR C N 10









