Alkynes
advertisement

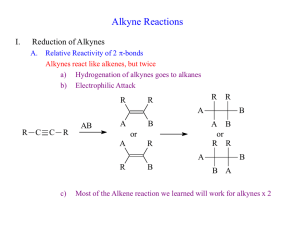
Alkynes A. Structure Suggested Reading: Chapter 8 in McMurry Suggested Problems: 8.1, 8.3-8.14, 8.19-8.47 B. Nomenclature C. Reactions 1. Addition of HX (geminal dihalide) 2. Addition of X2 (tetrahalide) 3. Hg2+ Catalyzed Hydration 4. Hydroboration/Oxidation 5. Reduction a. alkane b. cis-alkene c. trans-alkene 6. Oxidative Cleavage 7. Alkylation Reactions D. Retrosynthetic Analysis ALKYNES! H HO O H3C C C C C C C C H3C C C C C C Capillin (fungicidal activity) C Ichthyothereol H O (convulsant used by Amazon Indians for poisoned arrowheads) H3C OH CH C Norquen Ovastol (in oral contraceptives) H3CO 1 Alkyne Nomenclature 1. Follow alkene rules, but use -yne as suffix. 2. If more than one triple bond is present, use -diyne, -triyne, tetrayne . . . 3. If both double and triple bonds are present, use -enyne as suffix Number from side with nearest multiple bond (either double or triple) If a double and triple bond is equidistant, make the double bond the lower number H3C C C CH3 Br CH3 H2 H3C C C C C C CH3 H H 2-butyne 6-bromo-2-methyl-3-heptyne CH3 HC C CH2CH2CH2CH2CH CH2 HC C CH2CHCH2CH2CH 1-octen-7-yne CHCH3 4-methyl-7-nonen-1-yne Alkynes: Common Names and Groups R C C H R C C R terminal alkyne internal alkyne C HC CH HC C CH3 acetylene methylacetylene H2 H2 C C CH2CH3 butyl C C CH2CH3 H H butenyl CH phenylacetylene C C CH2CH3 butynyl H2 C C C H propargyl 2 KetoKeto-Enol Tautomerism Tautomers - constitutional isomers that differ in the location of a H and a double bond Tautomerization - conversion between two tautomers H H O H H H R C CH3 acidic conditions R H O O H O O R C CH3 R C CH3 H H O H O H H O R H H O O R C CH3 H Enol (alkene and alcohol) H R Keto (favored) O R C CH3 H O H basic conditions H O H Addition of Halogens to Alkynes For Terminal Alkynes: (internal alkynes result in a mixture of products) R H H-X (1eq.) X = Cl, Br, I R X H H-X (1eq.) H X H geminal dihalide vinylic halide (E or Z) Markovnikov Carbocation intermediate R H X-X (1eq.) X = Cl, Br R X X H X H R C C H Markovnikov syn + anti addition Carbocation intermediate X-X (1eq.) X X R C C H X X trans-dihalide tetrahalide Markovnikov Markovnikov anti addition halonium ion intermediate 3 Hydration of Terminal Alkynes R H HO O H R C CH3 R Terminal alkynes R HgSO4, H2SO4, H2O H methyl ketone enol Markovnikov carbocation intermediate H 1. disiamylborane, THF 2. H2O2, -OH, H2O H OH R H enol Anti-Markovnikov Concerted syn addition B O H2 R C CH aldehyde Hydration of internal alkynes leads to a mixture of ketones under both conditions H Disiamylborane is typically used instead of BH 3 to sterically block the addition of a second borane to the same alkyne Reduction of Alkynes R C C R H2, Lindlar's catalyst H H2, Pd/C H Na or Li, NH3 H R R H R CH2CH2H R R cis trans Lindlar’s catalyst Deactivated (poisoned) catalyst Pd, CaCO 3, lead acetate, quinoline 4 Oxidation of Alkynes Internal Alkyne R C C R' 1. O3, 2. H2O or KMnO4, H3O+/H2O R OH O R' HO O Terminal Alkyne R C C H 1. O3, 2. H2O or KMnO4, H3O+/H2O R OH CO2 O 5