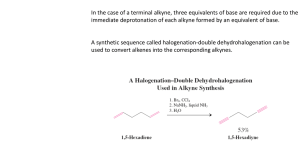
Alkyne Reactions I. Reduction of Alkynes R
advertisement

Alkyne Reactions I. Reduction of Alkynes A. Relative Reactivity of 2 p-bonds Alkynes react like alkenes, but twice a) Hydrogenation of alkynes goes to alkanes b) Electrophilic Attack R R R R A R C C R AB A B A B or R R or A B R A R c) B B B A Most of the Alkene reaction we learned will work for alkynes x 2 B. cis-Alkenes from Alkynes 1) Alkynes can be Hydrogenated under alkene conditions a) Pd/C or PtO2, 1 atm H2 Pd/C R C C R b) Gives saturated alkane product H R R H 2 2) H H H Lindlar Catalyst gives single H2 addition a) Lindlar Catalyst = 5% Pd-CaCO3, Pb(OAc)2, quinoline = b) Less active catalyst surface, so only one p-bond is added to c) H2 addition is syn (like for alkenes) and gives cis-alkene product C C H2, Lindlar catalyst o H H C C 25 C C. trans-Alkenes 1) Na is a strong reducing agent: Na Na+ + e2) Na dissolves in NH3(l) to give Na+ + e- (solvated electron) 3) Alkynes exposed to Na/NH3(l) are selectively reduced to trans-alkenes H R 1. Na, NH3(l) R C C R 2. H2O R H N 4) R C C R Na Mechanism: (see book for orbital picture) - R C C R H NH2 Alkyne Radical Anion R H C C- R H NH2 H II. C C R R trans-Alkenyl Radical R C R H C H Electrophilic Addition to Alkynes A. Alkynes are much like alkenes 1) p-bonds readily attacked by electrophiles 2) Terminal alkynes follow Markovnikov rule a) Electrophile ends up on less substituted Carbon b) Nucleophile ends up on more substituted Carbon N H E N R C C H R E Na R H C C R trans-Alkenyl Anion B. HX Additions to Alkynes 1) Single addition usually gives anti product H H3C C 2) CH3 H3C Br Br H H Br Br- Br H3C CH3 H Br Terminal Alkynes give Marknovnikov Addition Products H3C C 4) H Br BrH3C The second addition gives the Geminal Dihaloalkane (Markovnikov Rule) H 3) C CH3 CH3 C H HI -70 oC I CH3CI2CH3 (65%) + CH2 (35%) H3C Stopping the reaction after only one addition is difficult for terminal alkynes C. CH3CH2 C Halogenation of Alkynes 1) Anti addition of a single X2 molecule can be done to get vicinal dihaloalkane anti addition product 2) The second addition gives a tetrasubstituted product C CH2CH3 Br2, HOAc CH3CH2 Br C LiBr C Br Br2 CCl4 CH2CH3 CH3CH2CBr2CBr2CH2CH3 E-3,4-dibromo-3-hexene D. R C Ketones from Alkynes: Mercuric Ion Catalyzed Hydration 1) Like alkenes, Alkynes can be hydrated 2) The enol product undergoes tautomerization (interconversion of isomers by C=C, H shift) to give a ketone product H C R H2O, H+, HgSO4 H C R 3) 4) O C tautomerization H H C O C R Enol R The Enol and Ketone are called tautomers Ketone 2+ The Hg cation catalyzes the reaction; mechanism not understood yet R 5) The hydration step follows the Markovnikov Rule OH OH OH OH O + H2O, H , HgSO4 H H 6) Symmetric Alkynes give only 1 product HO C C H2O, H+, HgSO4 H O C C H C C H + H OH C C 7) Unsymmetric Alkynes give product mixtures HO + C C H O C C H2O, H , HgSO4 C C H + H H + OH O H C C C C H III. Anti-Markovnikov Additions to Alkynes A. Radical HBr additions 1) HBr adds to Alkynes by radical mechanism with radical initiator ROOR 2) Anti-Markovnikov Products due to need for most stable radical intermediate ROOR + HBr H3C C ROH + Br C H Br Br H3C C C H H Br 2 C C H Aldehydes from Hydroboration-Oxidation of Alkynes 1) Like with alkenes, BHR2 adds to less substituted side of an alkyne 2) R groups prevent boration of both p-bonds 3) Oxidation results in the enolaldehyde tautomerization HB H3C C H3C H B. Br C H H THF H C H3C C BR2 H H2O2 OH- C H3C H H H C OH Enol C H C H3C Aldehyde O IV. Alkenyl Halide Chemistry Alkenyl Halides don’t do SN1 or SN2 reactions 1) Alkenyl Halides preferentially eliminate to alkynes instead of substituting 2) Need strong base to get elimination, other nucleophiles give N.R. 3) Simple nucleophiles don’t give the substitution products 4) Alkenyl cation that would form is very unstable A. H H2C C SN 2 Br B. R IH2C C NO REACTION R H2O SN 1 Br H2C C too high energy to exist Alkenyl Organometallics can form and act as nucleophiles O H H2C C Br Mg THF H H2C C 1. H + MgBr 2. H2O, H H2C C CH3 C H3C OH V. Organocuprate Chemistry A. Most organometallic reagents don’t react with alkyl halides 1) Alkyl and Alkenyl Metal reagents don’t attack haloalkanes fast enough MgBr 2) + Alkynyl Metal reagents can react with haloalkanes CH nBuLi (base) THF, HMPA B. NO REACTION Br C- Br SN 2 Organocuprates are more reactive 1) Formation of Organocuprates 2 RLi + CuI H2C CHLi + CuI R2CuLi + LiI (CH2=CH)2CuLi Lithium Diethenylcuprate 2) Organocuprates will couple their R groups with Haloalkanes R2CuLi + R'X R R' C 3) 4) CH3I H3C 1. Li, Et2O 2. CuI H C H Either alkyl or alkenyl organocuprates work for the coupling reaction Along with the Alkynyl Anion reactions, we now can form many different C—C bonds 1. Li, Et2O C Cl 2. CuI CH nBuLi (base) THF, HMPA CH3CH3I (CH3)2CuLi H3C Et2O, 0 C H C H o CH3CH3I C 2 CuLi CH3CH2CH3 H3C C HMPA C- C CH2CH3 H Br SN 2 H C