THERMODYNAMICS REVIEW Thermodynamics required for compressible flow: First Law Energy Equation
advertisement

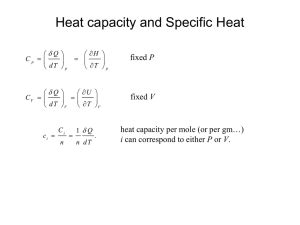
THERMODYNAMICS REVIEW Thermodynamics required for compressible flow: First Law Energy Equation Second Law Adiabatic, isentropic, constant entropy process Entropy Calculation Equation of State Ideal Gas Law First Law of Thermodynamics Observations: work can be transferr ed into heat more friction = more heat ∑ δQ ∝ ∑ δW Examples: rubbing you hands together friction in a wheel bearing braking a wheel Experiments: ∑ Q = C∑ W ∫ δQ = C∫ δW 1 BTU = 778 ft lbf 1 calorie = .427 kg m First Law for a Cycle ∫ δQ = ∫ δW ∫ (δQ − δW) = 0 The First Law is a Fundamental observation of nature, an axiom, which can not be proved but has never been found to be violated W First Law of Thermodynamics ∫ δQ = ∫ δW First Law for a Cycle ∫ (δQ − δW) = 0 Cycle A ⇒ B ∫ (δQ − δW) − ∫ (δQ − δW) = 0 Cycle A ⇒ C ∫ (δQ − δW) − ∫ (δQ − δW) = 0 Processes B and C ∫ (δQ − δW) − ∫ (δQ − δW) = 0 ∫ (δQ − δW) = ∫ (δQ − δW) ∫ (δQ − δW) is independent of path and A B A C B B 2 A B C C C 1 therefore a property. Define E as energy in all forms, KE + PE + U(T). 1 E1 − E 2 = ∫ (δQ − δW) = Q − W 2 First Law for a Processes Q = ∆E + W δQ = dE + δW Steady Flow Energy Equation Open, steady flow thermodynamic system - a region in space V Q p1 Wshaft p2 v2 v1 h2 V h1 Q = ∆E + W First Law Wflow in = ∫ pdV = p1 (V1 initial − V1 final ) = p1V1 = m p1v1 W = Wshaft + Wflow in + Wflow out = Wshaft + m p1v1 − m p 2 v 2 V2 E = u(T) + KE + PE = u(T) + + ρgh 2 V2 V2 Q = m(u1 + p1 v1 + + ρgh1 ) − m(u1 + p1 v1 + + ρgh1 ) + Wshaft 2 2 V2 + ρgh) + Wshaft Q = m∆(u + pv + 2 Steady Flow Energy Equation Open, steady flow thermodynamic system a region in space V2 Q = m∆(u + p v + + ρ g h) + Wshaft 2 for an adiabatic channel, diffuser or nozzle W = 0, Q = 0, without body forces since u + pv = h for an adiabatic flow V12 V22 h1 + = h2 + 2 2 for an nonadiabatic flow V12 V22 Q + h1 + = h2 + 2 2 IDEAL GAS CALCULATION What is the mass of 1.2 m 3 of oxygen at 24o C and a gage pressure of 500 kPa. Atmospheric pressure is 97 kPa p = p gage + p atmosphere = 500 kPa + 97 kPa = 597 kPa 8.314 kJ/kmole o K or kPa m3 /kmole o K R O2 = = .259813 kPa m3 /kg 32 pV 597 kPa ×1.2 m3 m= = = 9.28 kg RT .259813 kPa m3 /kg × 24o C + 273.16o K ( ) What is the volume of 1.2 lbm of air at 124o F and a gage pressure of 500 psia. Atmospheric pressure is 14.7 psia. p = p gage + p atmosphere = 500 psia + 14.7 psia = 514 psia 1545.15 lbf / lbm o R / lbmole ft lbf R air = = 53.336 28.97 lbm o R m R T 1.2 lbm × 53.336 ft lbf/ lbmo R × 124o F + 459.69o R V= = p 514 psia ×144 ft 2 /in 2 ( V = .5047ft3 ) Ideal Gas Law AVOGADRO'S LAW One (1) mole of any gas = 22.4 liters. 6.023×1023 molecules /mole of gas at STP (1 atm and 0o C ) BOLYES LAW p1 × v1 = p 2 × v 2 IDEAL (PERFECT) GAS LAW pv = RT pV = mRT p - absolute pressure, psia, kPa T - absolute temperatu re, o R, o K R* R= molecular weight lbf/lbm o R R = 1545.15 lbmole kJ kPa m 3 * R = 8.314 or kmole o K kmole o K * CHARLES LAW v1 T1 = v 2 T2 p1 T1 = p 2 T2 mass = moles × Molecular Weight m = n × Molecular Weight pv = R * T pV = nR * T % Error in assuming water is an ideal gas ∂T = JT Coefficient ∂p h h=constant PRINCIPAL OF CORRERSPONDING STATES COMPRESSIBILITY FACTOR Z Z is about the same for all gases at the same reduced temperature and the same reduced pressure where: P pv Z= RT pV Z= mRT PR = TR = p p critical T Tcritical ∂h cp = ∂T p=const ∂u cv = ∂T v=const ∆h = ∫ c p (T ) dT ∆u = ∫ c v (T ) dT Ideal Gas ∆h = c p ∫ dT SPECIFIC HEAT ∆u = c v ∫ dT h = u + pv pv = RT h = u + RT dh = du + RdT c p dT = c v dT + RdT cp − c v = R γR γ −1 R γ −1 = cv cp = with same units FOR IDEAL GAS ONLY IDEAL GAS IMPROVEMENTS h = ∫ c p (T )dT u = ∫ c v (T )dT Kelvin Planck Statement of the Second Law It is impossible to construct an engine which, operating in a cycle, will produce no other effect than the extraction of heat from a single reservoir and the performance of an equivalent amount of work. Clausius Statement of the Second Law It is impossible to have a system operating in a cycle which transfers heat from a cooler to a hotter body without work being done on the system by the surroundings Reversible Heat Engine Reversible Refrigerator Carnot Power Cycle Reversible constant t emperature heat trans fer, process 1 → 2, process 3 → 4 Reversible adiabatic expansion, process 2 → 3, process 4 → 1 Efficiency = Desired Effect W = Required Input Q in η CYCLE = QH − QL QH Carnot Principles 1. No engine operating between two heat reservoirs, each having a fixed temperature, can be more efficient than a reversible engine operating between the same reservoirs. ηactual ≤ ηCarnot 2. All reversible engines operating between two heat reservoirs, each having its own fixed temperature, have the same efficiency. 3. The efficiency of any reversible engine operating between two reservoirs is independent of the nature of the working fluid and depends only on the temperature of the reservoirs. 4. An absolute temperature scale can be defined in a manner independent of the thermometric material. Q1 T1 = Q 2 T2 Copyright © The McGraw-Hill Companies, Inc. Permi ssion required for reproduction or display. FIGURE 5-47 Proof of the first Carnot principle. 5-15 Thermodynamic Temperature Scale η = function(T1 , T3 ) η = 1− Q3 Q1 Q1 = function(T1 , T3 ) Q3 from engine schematics Q1 = f(T1 , T2 ) Q2 Q2 = f(T2 , T3 ) Q3 Q1 = f(T1 , T3 ) Q3 by identity, Q1 Q1 Q 2 = Q3 Q 2 Q3 substituting, f(T1 , T2 ) = f(T2 , T3 ) × f(T1 , T3 ) this equation can be satisfied only if, Qh Ql Th = Tl and Q l = Q h Tl Th δQ ∑T =0 dQ =0 ∫ T reversible processes Clasius Inequality ∫ δQ T ≤0 for a Reversible Cycle Q h − Q l Th − Tl = Q T h h T Q 1− l = 1− l Th Qh Qh Ql − =0 Th Tl δQ ∫ T =0 for an Irreversible Cycle Q h − Q l Th − Tl ⟨ Q T h h T Q 1− l ⟨ 1− l Th Qh Qh Ql − ⟨0 Th Tl δQ ∫ T ⟨0 Entropy Definition and Change δQ ∫ T ≤ 0 Clasius Inequality for a cycle composed of a reversible and an irreversible process 1 2 δQ δQ δQ = + ∫ T 2 irrev ∫ T 1∫rev T ≤ 0 DEFINE A PROPERTY S ENTROPY 1 δQ + S2 − S1 ≤ 0 ∫ T 2 irrev 1 δQ + ds ≤ 0 ∫ T 2 irrev 1 ds ≥ ds ≥ δQ ∫ T 2 irrev δQ ∫ T irrev δQ = ∫ Tds ds = δQ ∫ T rev 1 irreversible reversible 2 Entropy Change of an Ideal Gas δq = du + δw First Law δq = Tds Second Law Tds = du + pdv du pdv + ds = T T For an ideal gas : du = c v dT p R = T v dT dv +R ds = c v T v T v s 2 − s1 = c v ln 2 + R ln 2 T1 v1 h = u + pv dh = du + pdv + vdp du = dh − pdv − vdp Substituting into Tds = du + pdv Tds = dh − pdv − vdp + pdv Tds = dh - vdp dh v − dp ds = T T For an ideal gas , ds = c p dT dh = cp T T v dp dp = R T p dT dp −R T p T2 p2 s 2 − s1 = cp ln − R ln T1 p1 Isentropic Adiabatic Process pv γ = constant p1v1γ = p 2 v 2γ γ δQ = dU + δW First Law Adiabatic process δQ = 0 dU + δW = 0 p 2 v1 = p1 v 2 substitute from pv = RT c v dT + pdv = 0 T2 v1 = T1 v 2 for an ideal gas T= γ −1 p = 2 p1 γ −1 γ pv pdv vdp , dT = + R R R s 2 − s1 = c p ln T2 − Rln p 2 cv c pdv + v vdp + pdv = 0 R R c cv + 1pdv + v vdp = 0 R R 1 dp dv 1 =0 + 1 + _ 1 v 1 p γ − γ − integrating γ ln v + ln p = constant pv γ = constant Adiabatic Isentropic, ∆s = 0 IdealGas p1 T1 p s 2 − s1 = c p ln 2 p1 s 2 − s1 = c p γ −1 γ p − (c p − c v )ln 2 p1 p γ −1 p2 ln − (c p − c v )ln 2 γ p1 p1 p γ − 1 − (c p − c v ) s 2 − s1 = ln 2 c p γ p1 cp cv − p 2 c v c v s 2 − s1 = ln c p − (c p − c v ) = 0 cp p1 c v adiabatic process, pv γ = constant is also constant entropy ADIABATIC PROCESS γ Q=0, pv = constant, ∆s = 0 pvγ = constant p 2 v 2γ = p1v 2γ γ p 2 v1 = p1 v 2 substitute pv = RT T2 v1 = T1 v 2 γ −1 p2 = p1 γ −1 γ