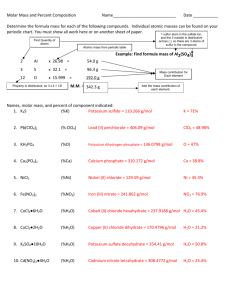
1 Chemistry 133 Problem Set Introduction
advertisement

1 Chemistry 133 Problem Set Introduction The following problem set includes all assigned problems for the Chemistry 133 Sections taught by R.H. Langley. The coding is the T.L. Brown, H. E. LeMay, Jr., B. E. Bursten, C. J. Murphy, P. M. Woodward and M. W. Stoltzfus, Chemistry the Central Science, 13th ed., Prentice Hall, 2012. Reference to this text will be “BLBMWS.” Chapter 1 1.1 Corresponds to BLBMWS Section 1.1 1.1 Define chemistry in your own words. 1.2 Make a list of all the physical properties you might observe when examining water in a glass. 1.3 Look at a pencil. (a) Make a list of as many of its extensive properties as you can. (b) Make a list of as many of its intensive properties as you can. 1.4 Which properties of a baseball will be the same on Earth as on Mars? 1.5 Define the terms mass and weight. Which of these terms is constant for an object? 1.6 (a) What could you do to increase the kinetic energy of a baseball? (b) What could you do to increase the potential energy of a baseball? 1.7 Define exothermic and endothermic processes and give an example of each. 1.8 What is the state of matter under normal conditions for each of the following? (a) gasoline (b) copper (c) nitrogen (d) sucrose (cane sugar) (e) helium 1.9 (a) Give three examples of substances that are gases under normal conditions. (b) Give three examples of substances that are liquids under normal conditions. (c) Give three examples of substances that are solids under normal conditions. 1.10 Describe each of the following as representing a chemical change or a physical change: (a) burning charcoal (b) cutting wood (c) iron rusting (d) changing water to ice (e) dissolving table salt in water. 1.11 Which of the following separations are based on physical properties and which are based on chemical properties? (a) Water is separated into hydrogen and oxygen. (b) A saltwater solution is separated into salt and water. (c) A sample containing small pieces of iron and sand grains is separated into sand and iron. (d) A sample of vodka is separated into alcohol and water. (e) Iron is separated from iron ore. 1.13 Which of the following changes involving water are physical changes and which are chemical changes? (a) Changing liquid water to steam (b) Converting liquid water to a mixture of hydrogen gas and oxygen gas (c) Dissolving table salt in water (d) Allowing ice to melt (e) Combining calcium oxide with water to produce calcium hydroxide 1.14 The gravity on the Moon is about one-sixth of that on Earth. (a) What would be the weight on the Moon of a piece of a NASA satellite weighing 60 lb on Earth? (b) If the satellite has a mass of 27 kg on Earth, what will be its mass on the Moon? 1.15 Classify each of the following as either an extensive or an intensive property: (a) volume (b) temperature (c) boiling point (d) density (e) mass. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 2 1.16 The following values were determined for a sample of water: mass = 125 g, volume = 0.125 L, density = 1.00 g/cm3, freezing point = 0.00°C and color = colorless. Classify each of these properties as extensive or intensive. Mass Extensive Volume Extensive Density Intensive Freezing Point Intensive Color Intensive 1.2 Corresponds to BLBMWS Section 1.2 1.17 What is the difference between a hypothesis, a theory, and a law? 1.18 What is the difference between observations and data? 1.19 Why is it important to control the different variables that may affect an experiment? 1.20 Does a serendipitous discovery invalidate the scientific method? Explain your answer. 1.21 A 5.0 g piece of aluminum wire was placed in a beaker containing 25.0 g of dilute sulfuric acid. The metal reacted with the acid to release hydrogen gas, which escaped into the atmosphere. After the reaction was complete, 28.9 g of material remained in the beaker. How many grams of hydrogen gas escaped? 1.1 g 1.22 The discovery of the element oxygen was in part based on the decomposition of a red solid known as mercuric oxide. This compound, when heated, decomposed to bright, silvery mercury metal, and colorless oxygen gas. Heating a mixture of mercury and oxygen at a lower temperature can regenerate the mercuric oxide. A 15.2-g sample of mercuric oxide is sealed in a 1.50-L container. The container plus sample had a total mass of 975.5 g. The container and contents are then heated until the mercuric oxide is completely decomposed. The container is then cooled and weighed. What is the final mass of the container, mercury, and oxygen? 1.3 Corresponds to BLBMWS Section 1.3 1.23 Define a pure substance and a mixture. Give an example of each. 1.24 Give two examples of each of the following. (a) an element (b) a compound (c) a homogeneous mixture (d) a heterogeneous mixture 1.25 What experiments could you perform to demonstrate that liquid water and ice contain the same compound? 1.27 Using the table inside the front cover of this textbook, list the symbol for each of the following elements: (a) hydrogen (b) lithium (c) aluminum (d) xenon (e) samarium (f) iron (g) copper (h) sodium (i) mercury (j) lead 1.28 Use the table inside the cover of this textbook, first, to classify each of the following elements as a metal, metalloid, or nonmetal, and, second, name the element: (a) K (b) Ra (c) Pt (d) Ne (e) Ge 1.29 Using the table inside the front cover of this textbook, identify each of the following elements from its symbol: (a) He (b) Kr (c) Ca (d) Cr (e) Dy (f) K (g) Ag (h) Sn (i) Sb (j) Au 1.30 Use the tables inside the front cover of this book to, first, classify each of the following elements as a metal, metalloid, or nonmetal, and, second, give the element’s symbol: (a) rubidium (b) rhenium (c) iodine (d) hydrogen (e) silicon 1.4 Corresponds to BLBMWS Section 1.4 1.32 Your roommate claims that the SI system is overly complicated because of all the different length measurements, such as meters, centimeters, millimeters, and kilometers. Outline how you would explain the fallacy of your roommate's claim about there being too many different units. 1.33 List the different SI base units and give an example of where each might be useful. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 3 1.34 Why is there no need for SI base units for area or volume? 1.35 You perform a calculation to determine the volume of a box. You enter the following information into your calculator: 12. × 12.0 × 12 and you report 1728 as the answer. It is very likely that your instructor will count the answer wrong. Why? 1.36 Identify the SI units that would be appropriate for measuring the following quantities: (a) the temperature of a glass of water (b) the volume of a bottle of a soft drink (c) the surface area of a football field (d) the distance from the Earth to the Moon (e) the speed of a bullet. 1.37 Which SI units would be reasonable choices for making the following measurements: (a) the thickness of a sheet of paper (b) the mass of your chemistry book (c) the temperature of the air on a hot, sunny day (d) the volume of the gas tank in your car (e) the speed you normally drive? 1.38 Give the numerical value for each of the following SI prefixes: (a) c (b) m (c) M (d) p (e) (f) d (g) k (h) a (i) T (j) n 1.39 Express each of the following units as a power of 10: (a) c (b) m (c) (d) d (e) k (f) a (g) G (h) n (i) M (j) p (a) c = 10–2 (b) m = 10–3 (c) = 10–6 (d) d = 10–1 (e) k = 103 (f) a = 10–18 (g) G = 109 (h) n = 10–9 (i) M = 106 (j) p = 10–12 1.40 Give the property measured by each of the following units: (a) °C (b) m (c) dm3 (d) km2 (e) ms 1.41 Give the property described by each of the following measurements: (a) 175 mm (b) 5.0 cm3 (c) 978 mg (d) 4.53 km2 (e) 273 K (a) Length (b) volume (c) mass (d) area (e) temperature 1.42 Classify each of the following as a time, temperature, mass, volume, or area measurement. (a) mm2 (b) mL (c) g (d) Ms (e) K 1.43 Which units of the English system could replace the following SI units? (a) 1.0 dm3 (b) 30.5 cm (c) 373 K (d) 220 kg/dm3 (e) 55 m/s? (a) 1.0 dm3 ft3 (b) 30.5 cm ft (c) 373 K °F (d) 220 kg/dm3 lbs/ft3 (e) 55 m/s? ft/s 1.5 Corresponds to BLBMWS Section 1.5 1.44 Define accuracy and precision. Give an example of a situation in which accuracy is high, but precision is low. Give an example of a situation in which accuracy is low, but precision is high. Hint: you may use a "bull's-eye" target to illustrate both situations. 1.45 Do the terms accuracy and precision apply to the conversion of 12 inches into feet? Why or why not? 1.46 How many significant figures are in Mega = 1 000 000? 1.47 Under what circumstances would it be correct to describe 1000 as having four significant figures? 1.48 Define a measured and an exact value. Give two examples of each. 1.49 Does an increase in the number of significant figures indicate an increase in precision or an increase in accuracy? 1.52 State, in your own words, how to determine if a zero is significant or not. 1.53 How can the addition of values result in a value that has more significant figures than any of the individual values has? Use 9.5 + 2.7 as your example. 1.54 How would you distinguish between a measured and an exact value? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 4 1.55 Determine which of the following quantities are measured and which are exact. (a) A book has a mass of 1.000 kg. (b) There are 1000 g in a kilogram. (c) There are 12 inches in a foot. (d) Thirty-seven people entered a room. (e) A football stadium holds 80,000 people. 1.56 How many significant figures are present in each of the following measurements? (a) 3842 kg (b) 4.27 × 10 2 s (c) 9.004735 ft (d) 0.000427 g (e) 34000 miles 1.57 Determine the number of significant figures in each of the following measurements. (a) 4.00 × 10 7 m (b) 8.0032 cm (c) 0.00353 kg (d) 3.24000 × 103 in (e) 12 atoms. 1.58 Round the following numbers to two significant figures: (a) 11.3 (b) −122 (c) 10.00 (d) 2000 (e) 5.280 × 10 3. 1.59 Round the following numbers to three significant figures: (a) 111.3 (b) −122.45 (c) 11.00 (d) 400000 (e) 6.580 × 106. 1.60 Perform the following calculations and round the answers to the appropriate number of significant figures: (a) 1.4837 + 1.48 (b) 432.32 − 4.32 (c) 2.52 × 48312 (d) 0.2235 / 19.3 (e) 4.226 + 8.227 + 1.0 1.61 Perform the following operations and round the answers to the appropriate number of significant figures: (a) 1.3827 g + 1.46 g (b) 412.32°C − 12.32°C (c) 3.52 ft × 5312 ft (d) 0.3235 g/ 22.5 cm 3 (e) 3.726 in + 5.427 in + 2.0 in. 1.62 Perform the following calculations and round the answers to the appropriate number of significant figures: (a) (14.32 / 2.54) − 3.2 (b) 4.854 + (3.33 • 45321) (c) 300 • (4.31 + 5.85) (d) 2.54 × 10 3 + [(8.55 × 10–2) • (5.88 × 104)] (e) 5.88 • (4.81 − 2.81). 1.63 Perform the following calculations and round the answers to the appropriate number of significant figures: (a) (24.32 cm2/ 2.54 cm) − 8.2 cm (b) 3.754 ft2 + (3.33 ft • 45321 ft) (c) 500 km • (2.31 km + 50.65 km) (d) 2.54 × 103 m2 + [(8.55 × 10–2 m) × (5.88 × 104 m)] (e) 6.28 cm • (5.11 cm − 3.11 cm). (a) 1.4 cm (b) 1.51 × 105 ft2 (c) 30000 km2 (d) 7570 m2 (e) 12.6 cm2 1.6 Corresponds to BLBMWS Section1.6 1.65 The first law of mathematics is, "The answer has to look right." How does this apply to unit conversions? 1.66 What are the two conversion factors that can come from 1 mi = 5280 ft? 1.67 When converting from pounds to ounces, how do you know which term belongs in the denominator of the conversion factor? To eliminate pounds, it is necessary to divide by pounds. Therefore, pounds must be in the denominator. 1.68 When a person picks up a 1-lb piece of steel and says it is heavier than a 1-lb bag of feathers, what does she really mean? 1.69 The weight of a bowling ball on the Moon is about one-sixth of its weight on Earth. How does the density of a bowling ball on the Earth compare to the density of the same bowling ball on the Moon? 1.70 To determine the density of an object, you must know its mass and its volume. Identify each of these quantities (mass, volume, and density) as extensive or intensive. 1.71 Perform the following conversions: (a) 526 m to kilometers (b) 3.42 s to seconds (c) 954 ng to grams (d) 0.024 m3 to cubic centimeters (e) 7351 kg/m3 to grams per cubic centimeter. 1.72 Convert the following values: (a) 275 cm to meters; (b) 4.95 x 10 –3 kg to grams; (c) 25.0 cm2 to square meters; (d) 45 s to picoseconds; (e) 25°C to kelvins. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 5 1.73 Perform the following conversions: (a) 1251 mm to inches; (b) 1.25 gal to liters; (c) 55 mi/hr to meters per second; (d) 1.785 ft3 to cubic centimeters; (e) 3.52 × 103 gal/hr to liters per minute. (a) 49.25 in (b) 4.73 L (c) 25 m/s (d) 5.054 × 104 cm3 (e) 222 L/min 1.74 Carry out the following conversions: (a) 1.00 ft3 to cubic inches; (b) 45.3 ft2 to square meters; (c) 1760 lbs/m3 to kilograms per cubic meter; (d) 745 mL to cubic meters; (e) 186,000 mi/hr to meters per second. 1.75 The density of air at room temperature (25°C) is 1.11 × 10 −3 kg/L. How many grams of air are in a room measuring 12.1 m × 7.2 m × 2.0 m? 1.9 × 105 g 1.76 The density of air at room temperature (0°C) is 1.20 × 10 −3 kg/L. How many grams of air are in a room measuring 12.1 ft × 7.2 ft × 20.0 ft? 1.77 Highly toxic hydrogen cyanide (HCN) has many industrial uses. As little as 1.0 × 10 –5 g/L is cause for concern. Calculate the number of grams of HCN at 1.0 × 10–5 g/L is in a room measuring 25 ft × 22 ft × 8.0 ft. 1.78 Nickel tetracarbonyl is one of the most toxic substances known. The recommended maximum concentration in air is 1.0 × 10−3 g/L. At this maximum concentration, how many grams of nickel tetracarbonyl would be in a room that measures 25.0 ft × 15.5 ft × 8.0 ft? 1.79 During the 1920s and 1930s, the United States minted silver dollars known as Peace Dollars. Each Peace Dollar weighed 26.73 g and was 90.0 % silver (the remainder was copper). In 1925, the price of silver was 69 cents per troy ounce. (a) Determine the value of silver in a Peace Dollar in 1925. (b) In 1980, silver prices reached $20.80 per troy ounce. What was the value of the silver in 10 Peace Dollars? (c) The current price of silver is about $47.65 per troy ounce. How many Peace Dollars are required to supply $500.00 of silver? (1 troy ounce = 31.103 g) 1.80 Until 1933, the United States minted gold coins for general circulation. The highest denomination produced was the twenty-dollar gold piece known as the double eagle. By an act of Congress in 1849, each double eagle weighed 516 grains and was 0.900 fine (33.436 g and 90.0 % gold (the remainder was copper). In 1934, the United States increased the price of gold from $20.67 per Troy ounce to $35.00 per Troy ounce. (a) Determine the value of gold in a double eagle before 1934. (b) How much was the gold in 10 double eagle coins worth in 1934? (c) The current price of gold is $1789.85 per troy ounce. How many double eagle coins are required to supply $1000.00 of gold? (d) Jewelers express the purity of gold in karats. Pure gold is 24 karat (24 K). A sample that is 50 % gold is 12 K [(12/24) × 100% = 50 %]. What is the purity of a double eagle in terms of karats? (1 troy ounce = 31.103 g) 1.81 Antifreeze contains the compound ethylene glycol. This compound not only lowers the freezing point of water but also increases the boiling point of water. The density of ethylene glycol is 9.35 lb/gal, and the density of water is 62.5 lb/ft3. (a) Is the density of water greater than the density of ethylene glycol? (b) Convert the density of ethylene glycol and water to grams per milliliter. (c) Assuming the density of a mixture of water and ethylene glycol is simply the weighted average of the amounts of the two liquids mixed, what is the density in grams per cubic centimeter of a mixture containing 1.00 gal of ethylene glycol in 3.00 gal of water? (Hint: to determine the weighted average density, multiply each individual density by the fraction of the final solution that is that particular substance.) 1.82 A teabag contains about 0.23 oz of tea and is able to make a cup of strong tea. If you have 1.00 oz of tea, how many milliliters of strong tea can you prepare? (1 cup = 236.6 mL) 1.83 When you use 0.857 ounces of coffee beans, you are able to make about 12 cups of strong coffee. If you have 1.00 ounce of coffee beans, how many milliliters of strong coffee can you prepare? (1 cup = 236.6 mL) 3.3 × 103 mL 1.84 In the United States, the measurement of rainfall is typically in inches. How many liters of rain fall on an acre of land if 0.75 in of rain are measured? (1 square mile = 640 acres) 7.7 × 104 L © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 6 1.7 Summary 1.87 Louis Pasteur made many significant discoveries, one of which was pasteurization. This process consists of heating a substance, such as milk, to kill the bacteria responsible for spoilage. Pasteurization of milk requires a temperature of 145°F. Determine the pasteurization temperature for milk in degrees Celsius and in kelvins. 1.88 The pasteurization of beer utilizes heat to kill any microorganisms that could lead to spoilage. Normal pasteurization of beer is carried out at 135°F, but an alternative method, called flash pasteurization, utilizes a higher temperature of nearly 165°F for a shorter time. Convert the temperatures 135°F and 165°F to degrees Celsius. 1.8 Summary 1.95 Round the mass values to two significant figures, and round the length values to three significant figures. (a) 11.345 kg (b) 122.22 km (c) 10.7500 cm (d) 6000 g (e) 5.280 × 10 3 m. 1.96 A small beaker is weighed on a balance and found to have a mass of 25.48 g. When it is carefully filled to the rim with water and re-weighed, the mass is 55.78 g. A small piece of metal is then gently dropped into the filled beaker, causing a total of 1.55 g of water to overflow. The total mass of the beaker, the remaining water, and the metal is 68.02 g. The density of water is 1.00 g/mL. Determine the density of the metal. 7.90 g/mL 1.97 In 1994, the United States produced 89.20 billion pounds of sulfuric acid. The density of this sulfuric acid was 1.84 g/mL. (a) Determine the kilograms of sulfuric acid produced in 1994. (b) Determine the gallons of sulfuric acid produced in 1994, considering that 1 L = 1.057 qt. (c) Determine the cubic millimeters of sulfuric acid produced. 1.98 In 1991, the United States produced 11.70 billion pounds of ethylene dichloride. The density of this compound was 1.218 g/mL. (a) Determine the kilograms of ethylene dichloride produced in 1991. (b) Determine the gallons of ethylene dichloride produced in 1991. (1 L = 1.057 qt) (c) Determine the cubic millimeters of ethylene dichloride produced. 1.99 Many of the units in the English system are no longer in common use. Some examples are the palms (0.1666667 cubits), the rood (40 square perches), and the tun (4 hogsheads). (a) A cubit is 1.5 ft. How many centimeters are in 10.5 palms? (b) A square perch is 30.25 yd 2. How many square millimeters are in 3.25 roods? (c) A hogshead is 63 gal. How many cubic centimeters are in 4.2 tuns? 1.100 You want to determine the density of a colorless liquid in a small bottle. You weigh the bottle and liquid and find the total mass is 25.45 g. You then pour the liquid into a graduated cylinder and determine the volume to be 10.2 mL. Finally, you weigh the empty bottle and find its mass to be 9.45 g. What is the density of the colorless liquid in g/mL? 1.102 You have an irregular shaped sample of an unknown metal. The sample weighs 15.68 g. To determine the volume of the sample, you carefully place it in a graduated cylinder that already contains 5.5 mL of water. The volume of the water plus metal in the graduated cylinder is 7.0 mL. Determine the density of the metal. 1.104 Calculate the thickness, in micrometers, of a piece of gold foil, given the following information: width = 3.27 inches, length = 0.51 inches, mass = 1.66 × 10 –3 g, density = 19.3 g/cm3. 0.080 m 1.105 Aluminum metal has a density of 2.70 g/cm3. Calculate the thickness, in inches, of a piece of aluminum foil weighing 2.87 g and measuring 3.00 × 10 2 mm by 0.500 m. Chapter 2 2.1 Corresponds to BLBMWS Section 2.1 2.1 What is an atom? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 7 2.2 Who receives credit with first suggesting the existence of atoms? 2.3 List the basic postulates of Dalton's atomic theory. Which of these postulates required modification since Dalton's time? 2.4 How does Dalton's atomic theory explain the law of definite composition? 2.5 How does Dalton's atomic theory explain the law of multiple proportions? 2.6 (a) What was the first subatomic particle to be discovered? (b) Which was the last of the basic subatomic particles discovered? 2.7 Describe Rutherford's experiment that discovered the nuclear nature of the atom. 2.8 A sample of carbon dioxide from a burning piece of charcoal contains 27% carbon and 73% oxygen. A different sample of carbon dioxide also contains 27% carbon and 73% oxygen. The percentages are the same for all samples analyzed. Explain these observations with respect to atomic theory. 2.9 The analysis of a sample of bottled water found it to contain 89% oxygen and 11% hydrogen. An Antarctic expedition brought back a sample of ice from the Ross Ice Shelf in Antarctica. The ice sample was melted and analyzed. The melted ice contained 89% oxygen and 11% hydrogen. How does the similarity in the composition of these two samples of water support atomic theory? 2.10 Samples from a series of four compounds containing manganese and oxygen have the following compositions: Compound Mass of Manganese (g) Mass of Oxygen (g) 1 24.6 7.2 2 25.4 11.1 3 33.7 19.6 4 38.5 39.2 (a) Calculate the grams of oxygen per gram of manganese in each compound. (b) How do your answers support atomic theory? 2.11 Iron forms a variety of compounds with oxygen. The analyses of three of these compounds appear in the following table. Compound Mass of Iron (g) Mass of Oxygen (g) 1 24.6 10.6 2 25.4 7.29 3 33.7 12.9 (a) Determine the grams of oxygen per gram of iron in each of the compounds. (b) Explain how your answers relate to atomic theory. 2.12 Glucose (blood sugar) combines with oxygen in your body to produce carbon dioxide, water, and energy. A sample of glucose weighing 18.0 g combines with 19.2 g of oxygen to produce 26.4 g of carbon dioxide. How many grams of water formed? 2.13 Carbon monoxide, CO, reacts with iron(III) oxide, Fe 2O3, in a blast furnace to produce carbon dioxide, CO2, and iron, Fe, metal. A blast furnace loaded with 45 tons of iron(III) oxide produced 32 tons of iron and 37 tons of carbon dioxide. How many tons of carbon monoxide reacted? 24 tons carbon monoxide 2.2 Corresponds to BLBMWS Sections 2.2-2.3 2.14 (a) Which subatomic particle(s) is/are in the nucleus of an atom? (b) Which sub-atomic particle(s) is/are outside the nucleus of an atom? 2.15 List the subatomic particles in order of decreasing mass. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 8 2.16 Why is it preferable to use the number of protons instead of the number of electrons to identify the atomic number of an atom? 2.17 (a) How is it possible for atoms of different elements to have the same mass number? (b) Is it possible for atoms of different elements to have the same atomic number? Explain. 2.18 Two forms of oxygen occur in nature. There is the oxygen gas we breathe, and there is ozone gas in the upper atmosphere and in certain types of air pollution. Are the two types of oxygen isotopes or allotropes? Explain. 2.19 (a) Why does removing electrons produce an ion with a positive charge (a cation)? (b) Why does adding electrons produce an ion with a negative charge (an anion)? 2.20 One of your classmates looks on the periodic table and finds the number 35.45 associated with the element chlorine, Cl. Your classmate then states that 35.45 is the mass number of chlorine. Explain the error in your classmate's statement. 2.21 A xenon atom has a radius of about 2.2 Å. (a) What is the radius of a xenon atom in picometers? (b) How many xenon atoms would it take to produce a line 1.0 inch long? 2.22 A potassium atom has a diameter of about 5.6 Å. (a) What is the radius of a potassium atom in nanometers? (b) How many potassium atoms would it take to produce a line 1.0 yard long? 2.23 An oxygen-16 atom has a diameter of 3.0 × 102 pm, but the diameter of the nucleus is only 5.0 fm. A single oxygen-16 atom weighs 15.9949 amu, and its nucleus weighs 15.9905 amu. (a) How many oxygen atoms are necessary, when placed in a row, to yield a line 1.0 foot long? (b) How many oxygen nuclei are necessary to yield a similar row that is 1.0 foot long? (c) Determine the density of an oxygen-16 atom in g/cm3. (d) Determine the density of an oxygen-16 nucleus in g/cm3. The volume of the atom or nucleus may be determined by V = (4/3)r3. 2.25 Determine the number of protons, electrons, and neutrons in each of the following atoms: (a) 58 26 Fe (d) (a) (b) (c) (d) (e) 110 48 Cd (e) Protons 6 19 26 48 109 268 109 11 6 C (b) 40 19 K (c) Mt Electrons 6 19 26 48 109 Neutrons 5 21 32 62 159 2.26 Determine the number of protons, electrons, and neutrons in each of the following isotopes used in medicine: (a) chromium-51 (b) strontium-85 (c) gadolinium-153 (d) cobalt-60 (e) cobalt-57. 2.27 For each of the following isotopes, determine the number of protons, electrons, and neutrons: (a) barium-137 (b) chlorine-35 (c) samarium-153 (d) zinc-60 (e) iridium-190. 2.30 Complete the following table describing the atoms of five elements: 31 131 Symbol ___ ___ ___ 15P 54Xe Protons ___ 57 ___ ___ ___ Electrons ___ ___ ___ 50 79 Neutrons ___ 80 ___ 82 ___ Mass number ___ ___ ___ ___ 197 2.31 Complete the following table describing the atoms of five atoms or ions: 32 Symbol ___ ___ ___ ___ 16S Protons ___ 30 ___ ___ 62 Electrons ___ 28 ___ 36 59 Neutrons ___ 35 126 ___ ___ Net charge 0 ___ 3+ 1– ___ Mass number ___ ___ 209 84 150 © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 9 2.32 Complete the following table describing the atoms of five atoms or ions: 81 55 3+ Symbol ___ ___ ___ 35Br 26Fe Protons ___ ___ 36 82 52 Electrons ___ ___ ___ ___ 54 Neutrons ___ ___ 50 ___ 76 Net charge ___ ___ 0 2+ ___ Mass Number ___ ___ ___ 207 ___ Symbol Protons Electrons Neutrons Net charge Mass Number 81 35Br 35 35 46 0 81 55 3+ 26Fe 26 23 29 3+ 55 𝟖𝟔 𝟑𝟔𝐊𝐫 𝟐𝟎𝟕 𝟐+ 𝟏𝟐𝟖 𝟐– 𝟖𝟐𝐏𝐛 𝟓𝟐𝐓𝐞 36 36 50 0 86 82 80 125 2+ 207 52 54 76 2– 128 2.33 Complete the following table describing the atoms of five atoms or ions: 81 55 Symbol ___ ___ ___ 36Kr 25Mn Protons ___ ___ 40 84 49 Electrons ___ ___ ___ ___ 46 Neutrons ___ ___ 51 ___ 66 Net charge ___ ___ 0 2– ___ Mass Number ___ ___ ___ 209 ___ 2.3 Corresponds to BLBMWS Section 2.4 2.34 Dalton stated that all atoms of an element are identical. Which of the following properties are identical for all the atoms of an element: mass number, atomic mass, atomic number? 2.35 (a) In general, combinations of what type of elements give ionic compounds? (b) Combinations of what type of elements give molecular compounds? 2.36 Is it possible for the molecular formula and the empirical formula to be the same? Explain why or why not. 2.37 There are two naturally occurring isotopes of silver: silver-107 and silver-109. Silver-107 has a mass of 106.90509 amu (abundance = 51.83%), and silver-109 has a mass of 108.9047 amu (abundance = 48.17%). Determine the atomic mass of silver from these data. 2.38 Silicon, Si, is the second most abundant element in the Earth's crust. This element has three naturally occurring isotopes. Their masses and abundances are as follows: silicon-28 (27.9769265 amu), 92.23%; silicon-29 (28.9764947 amu), 4.67%; and silicon-30 (29.9737702 amu), 3.10%. Determine the atomic mass of natural silicon. 2.39 There are five naturally occurring isotopes of the element germanium, Ge. The masses and the percent abundances of each of the isotopes are in the following list: Isotope Mass (amu) Abundance (%) Germanium-70 69.9243 20.5 Germanium-72 71.9217 27.4 Germanium-73 72.9234 7.8 Germanium-74 73.9219 36.5 Germanium-76 75.9214 7.8 Determine the atomic mass of germanium from these data. 72.6 amu © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 10 2.40 The noble gas krypton, Kr, consists of six natural isotopes. The masses and percent abundance for each of these isotopes are in the following table: Isotope Mass (amu) Abundance (%) Krypton-78 77.9204 0.35 Krypton-80 79.9164 2.25 Krypton-82 81.9135 11.6 Krypton-83 82.9141 11.5 Krypton-84 83.9115 57.0 Krypton-86 85.9106 17.3 Determine the atomic mass of krypton from these data. 2.41 The atomic mass of rubidium, Rb, is 85.4678 amu. Natural rubidium consists of two isotopes, rubidium-85, and rubidium-87. The mass of a rubidium-85 atom is 84.9117 amu, and the mass of a rubidium-87 atom is 86.9099 amu. (a) Determine the number of protons, electrons, and neutrons in each of the two rubidium isotopes. (b) Determine the percent abundance of each isotope. (a) Protons Electrons Neutrons Rubibium-85 37 37 48 Rubidium-87 37 37 50 (b) 27.83 % 87Rb 72.17 % 85Rb 2.42 The lanthanide element europium, Eu, consists of two natural isotopes. The lighter isotope is 151Eu, with a mass of 150.919846 amu, and the heavier isotope is 153Eu, with a mass of 152.921226 amu. The atomic mass of europium is 151.964 amu. (a) Determine the composition of each isotope in terms of protons, neutrons, and electrons. (b) Calculate the percent abundance of each of the two isotopes. 2.43 Determine the formula mass of each of the following to four significant figures. (a) O 2 (b) H2O (c) CO2 (d) Fe2O3 (e) NH4NO3 2.44 Calculate the formula mass for each of the following to three decimal places. (a) O3 (b) Fe3O4 (c) CCl4 (d) (NH4)2SO4 (e) C6H12O6 2.45 Convert each of the following molecular formulas to empirical formulas. (a) H 2C2O4 (b) N2O4 (c) C6H12O6 (d) Fe2O3 (e) B3N3H6 2.46 Convert the following empirical formulas to molecular formulas. The approximate molecular masses for each substance are in parentheses. (a) NO2 (90 amu) (b) CH2O (180 amu) (c) CH2O (120 amu) (d) MnSO4 (151 amu) (e) P2O5 (284 amu) 2.47 What information is necessary to convert a molecular formula to a structural formula? 2.48 The structural formula for dinitrogen oxide may be written as N=N-O. (a) What is the molecular formula of this compound? (b) Describe what information is present in the structural formula that is not in the molecular formula. 2.49 (a) What is the molecular formula for the compound shown in the following picture? (b) Is this likely to be an ionic or a covalent compound? H H H O C F H C C C C C F H H H H O H H © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 11 2.50 Determine the molecular formula of each of the following compounds. H O Cl H O (a) O (b) O H Cl O O Cl O H O Cl O O (c) (a) HClO (b) HClO2 (d) (c) HClO3 (d) HClO4 2.51 Determine the molecular formula of each of the following compounds. Cl H H C C H Cl H H H C N C H H H (a) S H F (c) H (b) F O H F F F F Br F F (d) 2.4 Corresponds to BLBMWS Section 2.5 2.52 Examine the periodic table in the front of the book. (a) How many periods are there? (b) How many groups are there? 2.53 (a) How many of the elements are nonmetals? (b) How many of the elements are metalloids? (c) How many of the elements are metals? 2.54 List three ways of distinguishing a metal from a nonmetal (assuming you do not know the name of the substance). Will these methods work when differentiating a metal from a metalloid? 2.55 Locate each of the following elements on the periodic table. Give the symbol for the element, and classify it as a metal, metalloid (semimetal), or nonmetal. (a) titanium (b) tellurium (c) tantalum (d) fluorine (e) bromine 2.56 Give the name and the symbol for each of the metalloids (semimetals) shown on the periodic table. 2.57 From the list provided here, choose the best match for each of the descriptions that follow – S, Na, As, I, Ba, Al, Fe, In, H, and Xe. Use each element only once. (a) Which forms ions with a 3+ charge? (b) Which forms ions with a 2– charge? (c) Which element is a metalloid? (d) Which element is a transition metal? (e) Which element is an alkaline earth metal? (f) Which element is most like aluminum? (g) Which element is a noble gas? (h) Which element is an alkali metal? (i) Which element is a halogen? (j) Which is the lightest nonmetal? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 12 2.58 From the list provided here, choose the best match for each of the descriptions that follow – S, Fr, Ge, At, Yb, Sr, Po, Bh, Tl, H, and Ne. Use each element only once. (a) Which element is a metalloid? (b) Which element is a transition metal? (c) Which element is an alkaline earth metal? (d) Which element is most similar to aluminum? (e) Which element is a noble gas? (f) Which element is an alkali metal? (g) Which element is a halogen? (h) Which is the heaviest member of the oxygen family? 2.5 Corresponds to BLBMWS Section 2.6-2.9 2.59 The most reactive of all the metals are the alkali metals, and the most reactive of all the nonmetals are the halogens. What type(s) of ions do you expect reactions of elements in these two groups to form? 2.60 Why is the compound MgCl unlikely to exist? 2.61 (a) One of your fellow students tells you that calcium ions, Ca 2+, will combine with nitride ions, N3–, to produce CaN. Explain why this is not the formula of the compound containing calcium and nitride ions. (b) Another of your fellow students wishes to make a compound containing ammonium ion, NH 4+, and sodium ions, Na+. Explain why this is not possible. (a) The total charge must be zero, CaN represents one Ca2+ and one N3–. These charges add as +2 –3 = –1 ≠ 0; therefore, the formula cannot be correct. (b) The total charge must be zero; it is impossible to add only positive charges together to get zero. 2.62 Based on the position of the elements on the periodic table, which of the following ions would you expect to be unlikely to form? (a) Ca3+ (b) O2– (c) Cl+ (d) Ba+ (e) N3– 2.63 Based on the position of the elements on the periodic table, predict the most likely charge exhibited by ions of each of the following elements. (Hint: look at the other elements in the same column.) (a) Rb (b) Ra (c) Ga (d) Se (e) At (a) Rb+ (b) Ra2+ (c) Ga3+ (d) Se2– (e) At– 2.64 Predict the formula of the compound formed when the following ions combine. (a) Sr 2+ and S2– (b) Fe3+ and O2– (c) NH4+ and NO3– (d) Al3+ and NO2– (e) Ca2+ and PO43– 2.65 The following ions may combine to form ionic compounds. Determine the simplest formula for each of the compounds that may form. (a) Se2– and Ca2+ (b) K+ and I– (c) Fe2+ and AsO43– (d) NH4+ and PO43– (e) Zr4+ and SO42– 2.66 Predict the formula of the compound formed by each of the following pairs of elements assuming that each element forms an ion with the charge predicted from its position on the periodic table. (a) K and Br (b) Ba and Se (c) Mg and N (d) Ca and I (e) Al and Cl 2.67 Predict the charge on ions formed by each of the following elements. Then fill the table with the formulas of the ionic compounds that may form from the ions. Nonmetallic Element__________ Metallic Element F O N K Mg Al _____ _____ _____ _____ _____ _____ _____ _____ _____ 2.68 Classify each of the following as an ionic compound or as a molecular compound. (a) NaCl (b) C 6H12O6 (c) CO2 (d) YF3 (e) RaCl2 2.69 Given the elements Mg, Cl, As, Cs, and Se, predict which will form an ionic compound with sulfur, S, and which will form a molecular compound with sulfur. 2.70 Classify each of the following as an organic or as an inorganic compound: (a) H 2O (b) C6H12O6 (c) Na2CO3 (d) HC2H3O2 (e) CO2 2.71 Which of the following compounds are organic? (a) CH 2O (b) (NH4)HCO3 (c) CS2 (d) C2H5OH (e) CaC2 © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 13 2.72 Determine the molecular formula and give the name of each of the following compounds. H O S Cl H (a) F S Cl (b) Cl F O I F (c) I (d) 2.73 Determine the molecular formula and give the name of each of the following compounds. F H S F S F H F (b) (a) Cl F Br F (c) F Cl Cl P Cl Cl (d) 2.6 Corresponds to BLBMWS Section 3.1 2.74 Define the terms reactants and products. 2.75 Which side of a reaction arrow has the products? Which side has the reactants? 2.76 How does a balanced chemical equation obey the law of conservation of matter? 2.77 List the reactants and products in the following equation, and tell what the terms in parentheses mean. Hg(l) + 2 H2SO4(aq) HgSO4(s) + SO2(g) + 2 H2O(l) 2.79 Historically, geologists often ran the following reaction to determine if a rock sample contained the mineral calcite (CaCO3). CaCO3(s) + 2 HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) The formation of carbon dioxide (CO2(g)) indicates the presence of calcite. List each of the reactants and products, and tell what the abbreviations in parentheses mean. 2.80 A student balances the equation H2(g) + Cl2(g) HCl(g) as H(g) + Cl(g) HCl(g) Explain why the final "balanced" equation is wrong. 2.81 One of your fellow students balances the following equation S8(s) + 8 O2(g) 8 SO2(g) Their answer was S(s) + O2(g) SO2(g) How would you explain to the student what they did wrong? S8 represents a group, not eight individual S atoms. The group must stay together. In general, never change a formula when balancing an equation. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 14 2.82 (a) What is the scientific basis (or law) exhibited by a balanced chemical equation? (b) How do the meanings of 8 S and S8 differ? 2.83 (a) An unbalanced chemical equation violates which scientific principle (or law)? (b) How do the meanings of 4 P and P4 differ? 2.84 How do the compounds NO, NO2, and N2O3 illustrate the law of multiple proportions? 2.7 Summary 2.87 How did the mass spectrometer further the understanding of atomic structure? 2.92 Fluorine was proposed as a possible element for use as the basis of atomic masses. The current atomic masses may be converted to another elemental basis by multiplying each atomic mass by a ratio of the new standard’s ideal atomic mass to the new standard element’s previous, carbon-12-based mass. For fluorine, the ratio would be 19.0000/18.9984. Determine the atomic masses for the following elements on a fluorine-based table. Also, determine the differences between the old and new masses. (a) H (b) C (c) O (d) Fe (e) U. 2.9 Summary 2.94 Name each of the following compounds. (a) CO2 (b) PCl3 (c) Cl2O (d) N2O5 (e) SCl2 (f) XeF4 (g) IF7 (h) CCl4 (i) P4O6 (j) Cl2O7 2.95 Name each of the following compounds. (a) CO (b) BCl3 (c) I2O (d) P4O10 (e) SiF4 (f) KrF2 (g) BrF5 (h) TeCl4 (i) SeO3 (j) Br2O5 (a) Carbon Monoxide (b) Boron Trichloride (c) Diiodine Oxide (d) Tetraphosphorus Decoxide (e) Silicon Tetrafluoride (f) Krypton Difluoride (g) Bromine Pentafluoride (h) Tellurium Tetrachloride (i) Selenium Trioxide (j) Dibromine Pentoxide 2.96 What is the formula of each of the following compounds? (a) carbon monoxide (b) sulfur trioxide (c) disulfur dichloride (d) selenium tetrabromide (e) nitrogen dioxide (f) krypton difluoride (g) nitrogen trichloride (h) diphosphorus pentasulfide (i) boron triiodide (j) dibromine pentoxide (a) CO (b) SO3 (c) S2Cl2 (d) SeBr4 (e) NO2 (f) KrF2 (g) NCl3 (h) P2S5 (i) BI3 (j) Br2O5 2.97 What is the formula of each of the following compounds? (a) silicon dioxide (b) diphosphorus trioxide (c) dinitrogen tetroxide (d) sulfur tetrabromide (e) carbon dioxide (f) xenon difluoride (g) phosphorus trichloride (h) disulfur decafluoride (i) boron trifluoride (j) diboron tetroxide 2.98 What is the formula of each of the following compounds? (a) hydrochloric acid (b) hydrogen sulfide (c) ammonia (d) sulfur dioxide (e) dinitrogen pentoxide (f) dichlorine trioxide (g) carbon dioxide (h) tetraphosphorus decaoxide (i) sulfur trioxide (j) diiodine oxide 2.101 Sulfur forms two compounds when it reacts with oxygen. In one compound, there is 1.00 g of oxygen for every gram of sulfur, and in the other compound, there are 1.50 g of oxygen for every gram of sulfur. Show how these compounds illustrate the law of multiple proportions. 2.102 What information is necessary to convert an empirical formula to a molecular formula and then to a structural formula? 2.105 The atomic mass of antimony, Sb, is 121.760 amu. Natural antimony consists of two isotopes, antimony-121, and antimony-123. The mass of an antimony-121 atom is 120.9038 amu, and the mass of an antimony-123 atom is 122.9041 amu. (a) Determine the percent abundance of each isotope. (b) A sample of antimony from another planet contained 24.35 % antimony-121 and 75.65 % antimony. What was the atomic mass of the antimony from this other planet? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 15 2.106 Phosphorus forms two compounds when it reacts with oxygen. In one compound, there are 1.00 g of phosphorus for every 1.29 g of oxygen, and in the other compound, there are 1.00 g of phosphorus for every 0.774 g of oxygen. Show how these compounds illustrate the law of multiple proportions. 2.107 Read the story Omnilingual by H. Beam Piper and comment on its applicability to information presented in this chapter. (This story is available on-line as an ebook through Project Guttenberg.) Chapter 3 3.2 Corresponds to BLBMWS Section 3.1 3.4 Which fundamental law(s) require that a chemical equation be balanced? 3.5 How does the listing of the phases affect the balancing of an equation? 3.6 During a study session, one of your friends takes the following equation: H2(g) + O2(g) H2O(l) Your friend balances the equation as: H2(g) + O2(g) H2O2(l) How would you explain the error your friend made? 3.7 In a study group reviewing for a chemistry exam, you ask your fellow students to balance the following equation: Al(s) + O2(g) Al2O3(s) One member of your group submits the following answer: 2 Al(s) + 3/2 O2(g) Al2O3(s) Explain why this answer is only partially correct. 3.8 You are helping a friend to understand why their grade on a recent chemistry exam was lower than expected. One of the questions on the exam asked for the following equation to be balanced: Fe3O4(s) + O2(g) Fe2O3(s) Your friend answered: 2 Fe3O4(s) + O2(g) 3 Fe2O3(s) + O(g) How would you explain the error to your friend? 3.9 One of your friends asks you to check their homework. You notice that one of their answers is: 6 Br2(l) + 12 KOH(aq) 10 KBr(aq) + 2 KBrO3(aq) + 6 H2O(l) You explain to your friend that this answer is not completely correct. What important detail about balancing equations did your friend forget? 3.10 Balance the following chemical equations by placing appropriate coefficients in the blanks: (a) ___ Cl2O5(g) + ___ H2O(l) ___ HClO3(aq) (b) ___KClO3(s) ___KCl(s) + ___O2(g) (c) ___SiCl4(l) + ___H2O(l) ___H4SiO4(aq) + ___HCl(aq) (d) ___Li3N(s) + ___H2SO4(aq) ___Li2SO4(aq) + ___(NH4)2SO4(aq) (e) ___C8H18(l) + ___O2(g) ___CO2(g) + ___H2O(l) (a) ___ Cl2O5(g) + ___ H2O(l) _2__ HClO3(aq) (b) _2__KClO3(s) _2__KCl(s) + _3__O2(g) (c) ___SiCl4(l) + _4__H2O(l) ___H4SiO4(aq) + _4__HCl(aq) (d) _2__Li3N(s) + _4__H2SO4(aq) _3__Li2SO4(aq) + ___(NH4)2SO4(aq) (e) _2__C8H18(l) + _25__O2(g) _16__CO2(g) + _18__H2O(l) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 16 3.11 Balance the following chemical equations by placing appropriate coefficients in the blanks: (a) ___ I2O5(s) + ___ H2O(l) ___ HIO3(aq) (b) ___ KNO3(s) ___ KNO2(s) + ___ O2(g) (c) ___ PCl5(l) + ___ H2O(l) ___ H3PO4(aq) + ___ HCl(aq) (d) ___ CaC2(s) + ___ H2O(l) ___ Ca(OH)2(s) + ___ C2H2(g) (e) ___ C2H6(g) + ___ O2(g) ___ CO2(g) + ___ H2O(l) 3.12 Balance the following chemical equations by placing appropriate coefficients in the blanks: (a) ___Yb2O3(s) + ___H3PO4(l) ___YbPO4(s) + ___H2O(l) (b) ___WO2(s) + ___O2(g) ___WO3(s) (c) ___P4O10(s) + ___H2O(l) ___H3PO4(aq) (d) ___C2H5OH(l) + ___O2(g) ___CO2(g) + ___H2O(g) (e) ___Mn(OH)3(s) + ___H2SO4(aq) ___Mn2(SO4)3(aq) + ___H2O(l) 3.13 Balance the following chemical equations by placing appropriate coefficients in the blanks: (a) ___ ZrO2(s) + ___ H3PO4(l) ___ Zr3(PO4)4(s) + ___ H2O(l) (b) ___ Fe3O4(s) + ___ O2(g) ___ Fe2O3(s) (c) ___ POCl3(l) + ___ H2O(l) ___ H3PO4(aq) + ___ HCl(aq) (d) ___ C4H4S(l) + ___ O2(g) ___ CO2(g) + ___ H2O(l) + ___ SO2(g) (e) ___ Pr2O3(s) + ___ H2SO4(aq) ___ Pr2(SO4)3(aq) + ___ H2O(l) 3.14 Balance the following chemical equations by placing appropriate coefficients in the blanks: (a) ___Al(s) + ___O2(g) ___Al2O3(s) (b) ___H3PO4(aq) + ___Fe(OH)2(s) ___Fe3(PO4)2(s) + ___H2O(l) (c) ___C6H14(l) + ___O2(g) ___CO2(g) + ___H2O(l) (d) ___N2O5(s) + ___H2O(l) ___HNO3(aq) (e) ___XeF2(s) + ___H2O(l) ___Xe(g) + ___HF(g) + ___O2(g) 3.15 Balance the following chemical equations by placing appropriate coefficients in the blanks: (a) ___ Al(s) + ___ F2(g) ___ AlF3(s) (b) ___ H3AsO4(aq) + ___ CuO(s) ___ Cu3(AsO4)2(s) + ___ H2O(l) (c) ___ C12H26(l) + ___ O2(g) ___ CO2(g) + ___ H2O(g) (d) ___ Rb2O(s) + ___ H2O(l) ___ RbOH(aq) (e) ___ XeF6(s) + ___ H2O(l) ___ XeO3(s) + ___ HF(g) (a) _2__ Al(s) + _3__ F2(g) _2__ AlF3(s) (b) _2__ H3AsO4(aq) + _3__ CuO(s) ___ Cu3(AsO4)2(s) + _3__ H2O(l) (c) _2__ C12H26(l) + _37__ O2(g) _24__ CO2(g) + _26__ H2O(g) (d) ___ Rb2O(s) + ___ H2O(l) _2__ RbOH(aq) (e) ___ XeF6(s) + _3__ H2O(l) ___ XeO3(s) + _6__ HF(g) 3.18 Determine the formulas for all the reactants and products for the following reactions. Then write balanced chemical equations for each reaction. (a) Carbon dioxide gas dissolves in liquid water to produce an aqueous solution of carbonic acid. (b) Solid potassium oxide reacts with liquid water to produce an aqueous solution of potassium hydroxide. (c) When it is heated, solid potassium nitrate decomposes to solid potassium nitrite and oxygen gas. (d) Hydrogen sulfide gas will dissolve in an aqueous solution of lead(II) nitrate, (Pb(NO3)2) to precipitate solid lead(II) sulfide (PbS) and form a dilute aqueous solution of nitric acid. (e) Solid dichlorine heptoxide forms an aqueous perchloric acid solution when dissolved in liquid water. 3.19 Determine the formulas for all the reactants and products for the following reactions. Then write balanced chemical equations for each reaction. (a) Gaseous sulfur dioxide dissolves in liquid water to produce an aqueous solution of sulfurous acid. (b) Solid barium oxide reacts with liquid water to produce an aqueous solution of barium hydroxide. (c) Liquid hydrogen peroxide decomposes when heated to produce liquid water and oxygen gas. (d) Carbon dioxide gas will dissolve in an aqueous solution of calcium hydroxide to produce solid calcium carbonate and liquid water. (e) Solid tetraphosphorus decaoxide dissolves in liquid water to from an aqueous solution of phosphoric acid. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 17 3.3 Corresponds to BLBMWS Section 3.3-3.4 3.22 Define Avogadro's number. (Note: 6.022 × 1023 is not the definition.) 3.23 Your study partner cannot understand how a mole of oxygen can contain the same number of particles as a mole of lead when the masses are so different. How would you explain that a mole of oxygen and a mole of lead have the same number of particles? You might want to compare a dozen eggs to a dozen pencils. 3.24 If nitrogen molecules weigh 28 amu each, without doing any calculations, how much does a mole of nitrogen molecules weigh? 3.25 What information is necessary to change the grams of a compound to the moles of that compound? Why is Avogadro's number not necessary? 3.26 What information is necessary to change the moles of a compound to the grams of a compound? Why is Avogadro's number not necessary? 3.27 When using moles or grams in a problem, what indicates that you also need to use Avogadro's number? 3.28 (a) How many carbon-12 atoms are in each mole of carbon-12? (b) How many grams does each mole of carbon-12 atoms weigh? (c) Calculate how many grams each carbon-12 atom weighs. 3.29 An atom of carbon-13 weighs 13.00335 amu. (a) How many carbon-13 atoms are in each mole of carbon-13? (b) How many grams does each mole of carbon-13 atoms weigh? (c) Calculate how many grams each carbon-13 atom weighs. 3.30 Morphine (C17H19NO3) is a powerful analgesic. (a) What is the molar mass of morphine? (b) How many grams does 2.00 mol of morphine weigh? (c) Calculate the number of moles of morphine present in a sample weighing 0.120 grams. (d) How many carbon atoms are present in 5.45 g of morphine? 3.31 The analgesic acetylsalicylic acid (aspirin) has the formula C9H8O4. (a) What is the molar mass of acetylsalicylic acid? (b) How many grams are present in 3.25 mol of acetylsalicylic acid? (c) How many moles of acetylsalicylic acid are present in a sample weighing 10.120 grams? (d) How many carbon atoms are present in 7.45 ng of acetylsalicylic acid? 3.32 Determine the number of grams present in each of the following samples. (a) 0.27952 mol of calcium chloride (CaCl2), (b) 4.37 × 1028 molecules of carbon dioxide (CO2), (c) 6.95 × 1015 molecules of water (d) 4.35 mol of carbon monoxide (CO), (e) 2.50 × 10 18 atoms of xenon (Xe). 3.33 Calculate how many grams each of the following samples weighs. (a) 4.3528 mol of krypton difluoride (KrF 2) (b) 3.75 × 107 molecules of sulfur dioxide (SO2) (c) 9.9527 × 1045 molecules of nitrogen oxide (NO) (d) 3.77 mol of sulfur hexafluoride (SF6) (e) 1 billion phosphorus atoms (P). (a) 530.14 g KrF2 (b) 3.98 × 10–15 g SO2 (c) 4.9592 × 1023 g NO (d) 551 g SF6 (e) 5 × 10–14 g P 3.34 Determine the number of molecules in each of the following samples: (a) 3.25 mol of aspirin (acetylsalicylic acid) (C9H8O4), (b) 0.0045 mol of table sugar (sucrose) (C12H22O11), (c) 4.55 g of water, (d) 2.3 ng of natural gas (methane) (CH4), (e) 3 kg of battery acid (sulfuric acid) (H2SO4). 3.35 Calculate the number of molecules present in each of the following samples. (a) 3.45 mol of hydrogen chloride (HCl) (b) 0.00525 mol of silicon dioxide (SiO2) (c) 15.3 g of silicon tetrachloride (SiCl4) (d) 14.3 mg of oxygen (O2) (e) 3.552 Mg of nitrogen (N2) 3.36 Determine the number of hydrogen atoms present in each of the following samples: (a) 10 molecules of water, (b) 0.672 mol of methane (CH4), (c) 1.32 mol of glucose (C6H12O6), (d) 2.6 g of urea ((NH2)2CO), (e) 3 ng of propane (C3H8). © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 18 3.37 Calculate the number of oxygen atoms that are present in each of the following samples. (a) 15 molecules of water (b) 0.552 g of carbon dioxide (CO2) (c) 3.93 mol of sulfur trioxide (SO3) (d) 3 g of dichlorine pentoxide (Cl2O5) (e) 47 cg of sulfur dioxide (SO2) 3.38 Nickel tetracarbonyl (Ni(CO)4) is used industrially in the purification of nickel. This compound is very toxic with as little as 6.7 × 10–9 g/L in the air being dangerous. (a) How many moles of nickel tetracarbonyl are present in a room 2.5 m × 3.7 m × 2.0 m? (b) How many molecules of nickel tetracarbonyl are in a liter? 3.39 Highly toxic hydrogen cyanide (HCN) has many industrial uses. As little as 1.0 × 10 –5 g/L is cause for concern. (a) Calculate the number of moles of HCN at 1.0 × 10 –5 g/L is in a room measuring 25 ft × 22 ft × 8.0 ft. (b) How many molecules of hydrogen cyanide, at 1.0 × 10 –5 g/L, are there per quart? (1 L = 1.057 qt) 3.40 The mineral spinel has the formula MgAl2O4. The mineral is very hard and is occasionally used as a semiprecious gemstone. The density of spinel is 3.581 g/cm3. Determine how many aluminum atoms are present in 7.500 cm3 of spinel. 2.274 × 1023 Al atoms 3.41 The mineral beryl has the formula Be3Al2Si6O18. The presence of small quantities of chromium, as an impurity, results in a gem known as emerald. The density of beryl is 2.640 g/cm3. Calculate the number of silicon atoms present in 4.250 cm3 of beryl. 3.42 (a) Calculate the number of moles of the following compound present in 15.25 grams. (b) How many molecules are present in 27.52 grams? F F C F O F C F F 3.43 (a) Calculate the number of moles of the following compound present in 12.50 grams. (b) How many molecules are present in 2.51 grams? H H H H H C C C H H C C H N H H H 3.4 Corresponds to BLBMWS Section 3.5 3.44 What is the definition of percent composition? Using C6H12O6 as an example, show how your definition applies to the percent carbon in this compound. 3.45 In addition to the chemical formula, what information is necessary to determine the percent composition of a compound? 3.46 For each of the following compounds calculate the percent by mass of the indicated element. (a) Carbon in octane (C8H18) a component of gasoline (b) Iodine in potassium iodide (KI) a compound used as a dietary supplement (c) Oxygen in methyl salicylate (C8H8O3) a compound used as wintergreen flavoring (d) Sodium in sodium stearate (NaC18H35O2) a compound in some soap (e) Nitrogen in ammonium nitrate (NH 4NO3) a compound used in some fertilizers 3.47 In each of the following, determine the mass percentage of the indicated element: (a) oxygen in water, (b) oxygen in hydrogen peroxide (H2O2) a substance sometimes used as a disinfectant, (c) nitrogen in urea ((NH 2)2CO) a compound sometimes used as a fertilizer, (d) hydrogen in isopropyl alcohol (rubbing alcohol) (C3H8O), (e) iron in hematite (an iron ore) (Fe2O3). © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 19 3.48 (a) Determine the percent by mass of each of the elements present in chlorophyll b (C 55H70MgN4O6). (b) Determine the percent by mass of each of the elements in hemoglobin (C2952H4664Fe4N812O832S8). (Yes the numbers are 2952, 4664, 4, 812, 832, and 8.) 3.49 Calculate the mass percent of each of the elements present in the following compound. H 52.144 % C H H C C H H O 13.13 % H H 34.728 % O 3.50 Calculate the mass percent of each of the elements present in the following compound. H H H C H O C H H 3.51 Polymers form by joining many small molecules together to produce gigantic molecules. An example of a polymer is polypropylene, which results from joining propylene molecules (C 3H6). A polypropylene molecule isolated at a certain factory has the formula C3027H6054. (a) Determine the molar mass of polypropylene. (b) Calculate the mass percentage of each of the elements in the polypropylene molecule. (c) Calculate the mass percentage of each of the elements in propylene. 3.52 The compound ajmalicine, isolated from various plant sources is an organic compound. Burning a sample of ajmalicine, weighing 1.0527 g, in oxygen produced 2.7605 g of CO 2, 0.6458 g of H2O, and 0.0837 g of N2. The compound contained C, H, N, and O. Determine the percentages of each of these elements. 3.53 Alstonine is a yellow solid isolated from several species of plants. A sample of alstonine weighing 1.0170 g sample was burned in oxygen. The reaction produced 2.6978 g of CO 2, 0.5259 g of H2O, and 0.0818 g of N2. The compound contained C, H, N, and O. Determine the mass percentages of each of the four elements present. 72.398 % C 5.787 % H 8.04 % N 13.78 % O 3.5 Corresponds to BLBMWS Section 3.5 3.54 Define both the empirical formula and the molecular formula. Are there any situations where the empirical formula and the molecular formula are the same? Give an example of a compound where the empirical and molecular formulas are the same. 3.55 During a study session, one of your fellow students takes the following information from an empirical formula calculation: 0.25 mol N and 0.50 mol of H, and reports the empirical formula to be N 0.25H0.50. Explain to your friend what they did wrong, and what the correct answer should be. 3.56 List the types of data that you may use to determine the empirical formula of a compound. 3.57 As you are working an empirical formula problem, you come up with the following mole values: C = 1.238, H = 4.000, N = 1.485 and O = 1.000. What is the empirical formula of this compound? Round each of the mole values to two significant figures, and then determine the empirical formula. Do your two answers agree? If your answers do not agree, what does this imply about rounding during empirical formula calculations? 3.58 Calculate the formula mass for each of the following: (a) H2O (b) Cl2O5 (c) (NH4)2CrO4 (d) Mg3(PO4)2 (e) CuSO4·5H2O 3.59 Determine the formula mass for each of the following: (a) CO2 (b) P4O10 (c) Zr(SO4)2 (d) (NH4)3PO4 (e) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 20 3.60 Convert each of the following to empirical formulas: (a) H 2O2 (b) C6H6 (c) CO2 (d) P4O10 (e) C6H12O6 3.61 Change each of the following molecular formulas to empirical formulas: (a) H 2C2O4 (b) C7H14 (c) P4O6 (d) N2O5 (e) B2H6 (a) HCO2 (b) CH2 (c) P2O3 (d) N2O5 (e) BH3 3.62 Three compounds, A, B, and C, were analyzed, and the following results were obtained. Convert these analyses to the empirical formulas for these three compounds. (a) Compound A contained 0.1248 moles of nitrogen and 0.2496 moles of hydrogen. (b) Compound B was found to contain 3.800 grams of iodine and 1.200 grams of oxygen. (c) Compound C was 39.2 percent phosphorus and 60.8 percent sulfur. 3.63 The analysis of three different oxides of chlorine gave the following information: Compound Moles Cl Moles O 1 3.255 6.510 2 2.786 1.393 3 1.332 3.330 Determine the empirical formula of each of the chlorine oxides. 3.64 Using the following data determine the empirical formulas for compounds A and B. (a) Compound A is 52.1 percent carbon, 13.1 percent hydrogen, and 34.7 percent oxygen. (b) Compound B contains 26.95 percent sulfur, 13.45 percent oxygen, and 59.60 percent chlorine. (a) C2H6O (b) SOCl2 3.65 In addition to carbon monoxide (CO) and carbon dioxide (CO 2), carbon forms two additional stable oxides. Analysis of samples of these two oxides gave the following information: Compound Percent C Percent O 1 52.97 47.03 2 50.02 49.98 Determine the empirical formula of these additional oxides of carbon. 3.66 (a) Glycine is an amino acid with a molecular mass of 75.07 amu. This amino acid is 32.00 percent carbon, 6.71 percent hydrogen, 18.66 percent nitrogen, and 42.63 percent oxygen. Determine both the empirical and molecular formulas for glycine. (b) Lysine is another amino acid with a molecular mass of 146.19 amu. This amino acid is 49.29 percent carbon, 9.65 percent hydrogen, 19.16 percent nitrogen, and 21.89 percent oxygen. Determine both the empirical and molecular formulas for lysine. 3.67 (a) Glucose is a sugar used by the body as the primary source of energy. This compound is blood sugar and has a molecular mass of 180.2 amu. This carbohydrate is 40.00 percent carbon, 6.714 percent hydrogen, and 53.28 percent oxygen. Calculate both the empirical and molecular formulas of glucose. (b) Ribose is a sugar that makes up part of the backbone of ribonucleic acid (RNA). This carbohydrate has a molecular mass of 150.1 amu. This sugar is 40.00 percent carbon, 6.714 percent hydrogen, and 53.28 percent oxygen. Calculate both the empirical and molecular formulas of ribose. (c) Determine the empirical formula of sucrose, cane sugar, if its molecular formula is C12H22O11. (d) The name carbohydrate implies the formula Cx(H2O)y. Do the empirical and molecular formulas of glucose, ribose, and sucrose have this implied formula? 3.68 The combustion of a 1.507 g sample of morphine (C17H19NO3) in pure oxygen produced carbon dioxide, water, and nitrogen gas. How many grams of each of the three products formed? 3.951 g CO2 0.9039 g H2O 0.07398 g N2 3.69 The combustion of a 0.0168 g sample of cocaine (C 17H21NO4) in pure oxygen produced carbon dioxide, water, and nitrogen gas. How many milligrams of each of the three products formed? 3.70 Hydrocarbons are compounds containing only hydrogen and carbon. A 2.00 mg sample of an unknown hydrocarbon was burned in oxygen. After combustion was complete, analysis of the products found 6.275 mg of carbon dioxide (CO2) and 2.569 mg of water (H2O). Determine the empirical formula of the unknown hydrocarbon. 3.71 In order to determine the formula of the compound carpiline a 0.5572 g sample was burned in oxygen. The reaction produced 1.5687 g of CO2, 0.2223 g of H2O, and 0.0384 g of N2. The compound contained C, H, N, and O. Determine the empirical formula. C26H18N2O3 © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 21 3.72 Isopentyl acetate is the substance that produces the characteristic odor of pears. A sample of isopentyl acetate weighing 3.75 × 10–3 g was burned in excess oxygen to produce 8.87 mg of carbon dioxide and 3.63 mg of water. Isopentyl acetate contains the elements carbon, hydrogen, and oxygen. Determine the empirical formula of isopentyl acetate. 3.73 Physostigmine salicylate has a variety of medicinal uses. In order to determine the formula of the compound physostigmine salicylate a 0.5560 g sample was burned in oxygen. The reaction produced 1.3020 g of CO 2, 0.3271 g of H2O, and 0.05654 g of N2. The compound contained C, H, N, and O. Determine the empirical formula. 3.74 Chlorophyll a is a magnesium-containing compound necessary for photosynthesis in plants. In order to determine the empirical formula for this compound the magnesium was first removed, and then 0.7693 g of the remaining material was burned in oxygen. The reaction produced 2.1423 g of CO 2, 0.5740 g of H2O, and 0.0496 g of N2. The compound contained C, H, N, and O. Determine the empirical formula. 3.75 At one time, the drug echitamidine was useful in the treatment of malaria. In order to determine the formula of the compound echitamidine a 0.6664 g sample was burned in oxygen. The reaction produced 1.7129 g of CO 2, 0.4558 g of H2O, and 0.0545 g of N2. The compound contained C, H, N, and O. Determine the empirical formula. 3.76 Many ionic compounds exist as hydrates. The mineral mirabilite is an example of a hydrate. The general formula for mirabilite is Na2SO4·xH2O. The value of x may be determined by calculating the difference in mass between mirabilite and sodium sulfate produced from a sample of this mineral. A 3.095 g sample of mirabilite is heated to 325°C to drive off the water. After the removal of the water, the sample was cooled and weighed. The dried sample weighed 1.364 g. Determine the value of x and the complete formula for mirabilite. 3.77 Copper(II) sulfate is normally isolated as a hydrate. The general formula for copper(II) sulfate hydrate is CuSO4•xH2O. A 4.355 g sample of copper(II) sulfate hydrate was heated to 150°C. At this temperature all the water was driven off the sample leaving anhydrous (without water) copper(II) sulfate. The anhydrous sample weighed 2.771 g. Determine the value of x in the formula of copper(II) sulfate hydrate. 3.78 Nickel(II) sulfate is normally isolated as one of two hydrates. The general formula for the nickel(II) sulfate hydrates is NiSO4•xH2O. From the following information determine the value of x in the formula of the two nickel(II) sulfate hydrates. (a) A 3.781 g sample of one of the nickel(II) sulfate hydrates was heated to 150°C. At this temperature all the water was driven off the sample leaving anhydrous (without water) nickel(II) sulfate. The anhydrous sample weighed 2.226 g. (b) A 4.843 g sample of one of the nickel(II) sulfate hydrates was heated to 150°C. At this temperature all the water was driven off the sample leaving anhydrous (without water) nickel(II) sulfate. The anhydrous sample weighed 2.669 g. 3.6 Corresponds to BLBMWS Section 3.6 3.79 Given the following chemical equation: 4 Sb(s) + 3 O2(g) 2 Sb2O3(s) List the six different mole ratios that this equation contains. 3.80 During a study session, your group attacks the following problem. Small quantities of very reactive chlorine gas may be produced in the laboratory by the following reaction: 4 HCl(aq) + MnO2(s) MnCl2(aq) + 2 H2O(l) + Cl2(g) (a) How many grams of greenish-yellow chlorine gas will form from the reaction of a solution containing 25.0000 g of hydrochloric acid, HCl, if the black solid manganese(IV) oxide is present in excess? (b) One member of the group comes up with the following solution: Grams Cl2 = = 48.6178 g Cl2 36 . 4606 g HCl 25.0000 g HCl 70.9054 g Cl 2 Upon checking the groups learns that the correct answer is 12.1545 g Cl 2. Where did your friend make their mistake? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 22 3.81 Methane (CH4) gas burns in oxygen (O2) gas according to the following equation: CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) Describe this reaction on a molecular, molar, an on a mass level. 3.82 Water, like many substances, will burn in fluorine gas. The equation for this reaction is: 2 H2O(l) + 2 F2(g) 4 HF(g) + O2(g) Describe this reaction on a molecular, molar, and on a mass level. 3.83 Aluminum (Al) metal reacts with hydrochloric acid (HCl) to form aluminum chloride (AlCl 3) and hydrogen (H2) gas. (a) Write a balanced chemical equation for this reaction. (b) Describe this reaction on a molecular, molar, and on a mass level. 3.84 Powdered aluminum will burn in oxygen gas to form solid aluminum oxide (Al 2O3). (a) Write a balanced chemical equation for the reaction of solid aluminum in oxygen gas to produce solid aluminum oxide. (b) Describe this reaction on a molecular, molar, and on a mass level. 3.85 LP gas is liquid propane (C3H8). The substance vaporizes and burns as follows: C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(l) (a) A 2.00 mol sample of propane is burned. How many moles of oxygen are required to burn this propane? (b) The combustion of 10.0 g of propane requires how many grams of oxygen? (c) Calculate the number of grams of oxygen required to burn 15.0 cm3 of LP gas. The density of LP gas is 0.5853 g/mL. (d) How many grams of carbon dioxide are produced when 15.0 cm3 of LP gas combusts? 3.86 The following reaction may be used to prepare iodic acid (HIO 3). I2(s) + 6 H2O(l) + 5 Cl2(g) 2 HIO3(aq) + 10 HCl(aq) How many grams of HIO3 could be prepared if 1.8460 g of iodine, I 2, 0.7870 g of H2O, and 2.5555 g of chlorine, Cl2 are mixed? 2.5360 g HIO3 3.87 One form of the element phosphorus has the formula P 4. This form is prepared by heating a mixture of calcium phosphate, sand (silicon dioxide), and coke (carbon) to 1400-1500°C. The reaction is: 2 Ca3(PO4)2(s) + 6 SiO2(s) + 10 C(s) 6 CaSiO3(l) + 10 CO(g) + P4(g) (a) Calculate the number of moles of silicon dioxide required to react with a 2.00 mol sample of calcium phosphate. (b) Calculate the number of grams of phosphorus formed from 3.50 mol of calcium phosphate. (c) Calculate the number of grams of calcium silicate that would form by the reaction of 125 g of calcium phosphate. 3.88 Oxalic acid (H2C2O4) reacts with potassium chlorate (KClO3) in the presence of an acid such as sulfuric acid (H2SO4) by the following reaction: H2C2O4 + H2SO4 + 2 KClO3 K2SO4 + 2 H2O + 2 CO2 + 2 ClO2 (a) How many moles of potassium chlorate are necessary to react with 1.50 mol of oxalic acid? (b) How many grams of sulfuric acid are necessary to prepare 2.45 mol of chlorine dioxide (ClO 2)? (c) How many grams of potassium sulfate (K2SO4) form when 125 g of potassium chlorate react with an excess of the other reagents. (d) If 25.8750 g of H2C2O4 are mixed with an excess of the other reactants, how many grams of ClO 2 are formed? 3.89 Rust deposits will dissolve in muriatic acid. Rust is mostly Fe2O3, and muriatic acid is an impure hydrochloric acid (HCl) solution. The acid will dissolve the rust to produce an aqueous solution of FeCl3 and water. (a) What is the balanced chemical equation for this reaction? (b) How many grams of rust could dissolve in a sample of muriatic acid containing 4.55 g of HCl? 3.90 The filament in an incandescent light bulb is made of tungsten metal (W). This metal is formed by heating yellow tungsten(VI) oxide (WO3) by reaction with hydrogen gas to produce not only pure tungsten metal but also water vapor. (a) Write a balanced chemical equation for this reaction. (b) How many grams of tungsten will 14.7 kg of hydrogen gas produce with excess tungsten(VI) oxide? (a) WO3(s) + 3 H2(g) W(s) + 3 H2O(g) (b) 4.47 × 105 g W © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 23 3.91 Chloroform (CHCl3) can be used as a sedative and as an anesthetic, but its use is not recommended because of toxicity issues. Chloroform may form during the chlorine treatment of water containing organic matter. A 1000.0 L sample of chlorine treated water was analyzed. Chemical treatment of the water freed the chlorine and allowed it to react with silver nitrate to form 0.0375 g of silver chloride (AgCl). How many grams of chloroform were in each liter of the treated water? 3.92 A 2.500 g pesticide sample, thought to contain DDT (C 14H9Cl5) was weighed. This sample was decomposed by reaction with sodium metal. Dissolving the sample in water gave a colorless solution. The addition of a silver nitrate solution to the colorless solution gave 0.0795 g of solid silver chloride (AgCl). What was the percent DDT in the sample? 1.57 % 3.93 A 5.782 g sample from a fish was thought to contain mercury. Analysis of the sample produced 6.7 × 10 –4 grams of mercury(II) sulfide (HgS). What was the percent mercury in the fish sample? 3.94 Chlorine is more reactive than bromine. The reactivity of chlorine allows it to displace bromine from compounds in reactions analogous to the following: 2 XBr3(s) + 3 Cl2(g) 2 XCl3(s) + 3 Br2(l) A 2.5178 g sample of XBr3 completely reacts with excess chlorine to produce 1.6357 g of XCl 3. (a) Determine the atomic mass of X. (b) What element is X? 3.95 Highly reactive fluorine gas (F2) will displace the less reactive chlorine gas from compounds. An example of this displacement is: 2 XCl3(s) + 3 F2(g) 2 XF3(s) + 3 Cl2(g) In one experiment, 2.7825 g of XCl3 reacted with an excess of fluorine gas to form 2.2835 g of XF 3. (a) Calculate the atomic weight of X. (b) What element is X? 3.96 One industrial method for the production of titanium involves the chlorination of the ore ilmenite (FeTiO 3) in the presence of carbon at 900°C. The compound TiCl4 distills away and reacts with magnesium metal to produce titanium metal. The unbalanced equation for the chlorination reaction is: FeTiO3(s) + C(s) + Cl2(g) TiCl4(g) + CO(g) + FeCl3(g) (unbalanced) (a) Balance the above equation. (b) How many kilograms of ilmenite are needed to prepare a metric ton (1000.0 kg) of TiCl4? (c) How many kilograms of ilmenite are needed to prepare a metric ton of TiCl 4 if the percent yield is only 85.2%? (d) Determine the theoretical yield of TiCl 4 from the reaction of 215 kg of ilmenite, 51.1 kg of carbon, and 331 kg of chlorine. (e) What is the percent yield in part (d) if only 115 kg of TiCl 4 formed? 3.7 Corresponds to BLBMWS Section 3.7 3.97 (a) What step must be added to a limiting reactant calculation that is not present in the previous stoichiometric calculations? (b) What is a simple way to recognize that a problem requires a limiting reactant calculation? 3.98 You are given the following equation: CuO(s) + 2 HCl(g) CuCl2(s) + H2O(g) You are then supplied with the following sets of data: i. 25.0 g CuO and excess HCl ii. 25.0 g of CuO and 75.0 g of HCl iii. 25.0 g of CuO and 39.0 g of CuCl2 iv. 25.0 g of CuO, 75.0 g of HCl, and 39.0 g of CuCl 2 Which of these four cases will involve the limiting reactant concept? 3.99 How much limiting reactant will remain after the reaction is complete? How is this different from the quantities of the other reactants? Are any values the same as when you started? 3.100 The last step in the manufacture of tricycles requires the assembly of a frame, a front wheel, a handlebar and two rear wheels. The manufacturer has on hand 1725 frames, 1700 front wheels, 1750 handlebars, and 3125 rear wheels. (a) Using only the parts on hand, how many tricycles may be assembled? (b) How many frames remain? front wheels? handlebars? rear wheels? (c) In terms of concepts introduced in this chapter, which tricycle part behaves as the limiting reagent? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 24 3.101 Chlorine dioxide (ClO2) is used as an industrial bleach. It is prepared commercially by the following reaction: 2 NaClO3(aq) + 2 H2C2O4(aq) 2 ClO2(g) + 2 CO2(g) + Na2C2O4(aq) + 2 H2O(l) (a) Calculate the number of grams of ClO2 formed from 525 g of NaClO3 and excess H2C2O4. (b) Calculate the number of grams of H2C2O4 needed to react completely with 525 g of NaClO3. (c) Calculate the number of grams of ClO2 formed when 475 g of NaClO3 and 375 g of H2C2O4 are mixed. (d) One of the reactants in part (c) was limiting and the other was in excess. How many grams of the excess remain after the reaction? 3.102 Small quantities of chlorine gas may be prepared in the laboratory by the following reaction: 2 H2SO4(aq) + MnO2(s) + 4 NaCl(aq) 2 Na2SO4(aq) + MnCl2(aq) + 2 H2O(l) + Cl2(g) In one experiment 1.2320 g of H2SO4, 0.5460 g of MnO2, and 1.4500 g of NaCl are mixed. How many grams of Cl 2 will form? 3.103 Chlorine is commonly used as bleach. Sodium thiosulfate (Na2S2O3) is used in the bleaching industry to destroy excess chlorine. Sodium thiosulfate is prepared by heating an aqueous solution of sodium sulfite, Na 2SO3, with sulfur. The reaction is: Na2SO3(aq) + S(s) Na2S2O3(aq) Calculate the maximum number of grams of sodium thiosulfate formed when 5.25 g of sodium sulfite are reacted with 7.25 g of sulfur. 3.104 The industrial bleach, chlorine dioxide (ClO2) is generated in small quantities through the following reaction. H2C2O4(aq) + H2SO4(aq) + 2 KClO3(aq) K2SO4(aq) + 2 H2O(l) + 2 CO2(g) + 2 ClO2(g) How many grams of ClO2 could form by the reaction of a mixture containing 8.3500 g of H 2C2O4, 9.0970 g of H2SO4, and 22.7200 g of KClO3? 3.105 Natural gas is primarily methane (CH4). Methane burns with the oxygen in air to produce carbon dioxide gas and water vapor. (a) Write a balanced chemical equation for the combustion of methane. (b) Calculate the maximum number of grams of water vapor that may form from the reaction of 15.2 g of methane with 15.2 g of oxygen. 3.106 The cyanide process was developed in the late nineteenth century for use in the gold and silver mining industry. Gold (Au) may be extracted from gold ore by the following reaction: 8 NaCN(aq) + 4 Au(s) + O2(g) + 2 H2O(l) 4 NaAu(CN)2(aq) + 4 NaOH(aq) How many grams of NaAu(CN)2 would form if 3.1000 g of Au, 1.5400 g of NaCN, 0.1350 g of O 2, and 0.1400 g of H2O are mixed? 3.107 Acetylene (C2H2) gas will react with liquid bromine (Br2) to produce liquid 1,1,2,2-tetrabromoethane (C2H2Br4). (a) What is the balanced chemical equation for this reaction? (b) If 180.0 g of bromine are combined with 30.0 g of acetylene, which one is the limiting reactant? (c) Determine the grams of acetylene, bromine, and 1,1,2,2-tetrabromoethane present after the reaction in part (b). 3.108 The following reaction may be used to prepare potassium iodate (KIO 3): 10 CrO3(s) + 3 I2(s) + 24 KCl(s) 6 KIO3(s) + 4 K3CrCl6(s) + 6 KCrO2(s) In one experiment, the following quantities of the reactants were mixed: 0.440 g of CrO3, 0.330 g of I2, and 7.500 g of KCl. How many grams of KIO3 formed? 3.8 Corresponds to BLBMWS Section 3.7 3.111 Write the definition of a percent yield. Why is the definition more useful than a simple chemical equation? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 25 3.112 You are given the following equation: CuO(s) + 2 HCl(g) CuCl2(s) + H2O(g) You are then supplied with the following sets of data: (a) 25.0 g CuO and excess HCl (b) 25.0 g of CuO and 75.0 g of HCl (c) 25.0 g of CuO and 39.0 g of CuCl2 (d) 25.0 g of CuO, 75.0 g of HCl, and 39.0 g of CuCl 2 (e) 25.0 g of CuO, 75.0 g of HCl, and 4.00 g of H 2O Which of these five cases has enough information to calculate the percent yield? In those cases where you can determine the percent yield, what is the actual yield in grams? 3.113 The compound butanal may be produced from 1-butanol by the following reaction: 1-butanol chromium(VI) butanal chromium(III) oxide chloride 3 CH3CH2CH2CH2OH(l) + CrO3(s) + 3 HCl(g) 3 CH3CH2CH2CHO(l) + CrCl3(aq) + 3 H2O(l) (a) How many grams of butanal may be formed by mixing 24.2 g of 1-butanol, 9.50 g of chromium(VI) oxide and excess hydrochloric acid (HCl)? (b) Determine the percent yield if only 18.5 g of butanal formed. 3.114 Small quantities of iron(III) chloride (FeCl3) may be prepared by the following reaction: 2 Fe3O4(s) + 3 Cl2(g) + 12 HCl(aq) 6 FeCl3(aq) + 6 H2O(l) + O2(g) What was the percent yield if 16.8295 g of FeCl3 were formed by reacting 15.2500 g of Fe3O4 with an excess of the other reagents? 3.115 Many detergents are derived from a group of organic compounds called sulfonic acids. Sulfonic acids are distinguished by the presence of the –SO2OH group. The following reaction may be used to produce laurlysulfonic acid, which may be converted to sodium lauryl sulfate for used in shampoos and detergents. C12H25SH(l) + 2 HNO3(aq) C12H25SO2OH(aq) + 2 NO(g) + H2O(l) (a) Calculate the theoretical yield of laurylsulfonic acid resulting when 177 g of C 12H25SH and 95.0 g of HNO3 are allowed to react. (b) Calculate the percent yield if only 158 g of laurylsulfonic acid formed. 3.116 Under certain conditions, the compound S4N4 may be explosive. One safe way to destroy this compound is by the following reaction: S4N4(s) + 6 NaOH(aq) + 3 H2O(l) Na2S2O3(aq) + 2 Na2SO3(aq) + 4 NH3(g) In one test reaction, 2.0000 g of S4N4 generated 0.5298 g of NH3. What was the percent yield in the test reaction? 71.66 % 3.9 Summary 3.121 The following reaction is important to the Haber process: N2(g) + 3 H2(g) 2 NH3(g) An industrial plant combines 13.5 metric tons of nitrogen gas and 4.0 metric ton of hydrogen gas in a Haber-Bosch reactor, after adjusting the conditions to optimum, 7.36 metric tons of ammonia form. What is the percent yield of ammonia? (A metric ton is 1000 kilograms.) 3.122 The Ostwald process begins with ammonia from the Haber-Bosch process through the following reaction: 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) An industrial plant combines 19.5 metric tons of ammonia gas and 37 metric tons of oxygen gas in a reactor, after adjusting the conditions to optimum, 9.50 metric tons of nitrogen oxide form. What is the percent yield of nitrogen oxide? (A metric ton is 1000 kilograms.) 3.123 The goal of the Ostwald process is to form nitric acid, HNO 3. This acid forms in the final step: 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) The percent yield of this reaction, in some cases, is 37.5%. Taking into account this percent yield, how many metric tons of nitric acid will form when 18.9 metric tons of nitrogen dioxide react with 1.75 metric tons of water? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 26 3.126 The following compounds may serve as nitrogen sources in fertilizer: calcium carbamate (Ca(NH2CO2)2), ammonia (NH3), calcium nitrate (Ca(NO3)2), and sodium nitrate (NaNO3). Determine the percent nitrogen in each of these substances. 3.127 Rank the following potential fertilizers from highest percent of nitrogen available to the lowest percent of nitrogen available. H N H H a O H N C N H c H N N H H b H H H Cl N Cl Cl d 3.10 Summary 3.128 Dicobalt octacarbonyl (Co2(CO)8) and copper(I) iodide (CuI) may be prepared by the following reaction: 2 CoI2 + 4 Cu + 8 CO Co2(CO)8 + 4 CuI (a) How many grams of CuI could be prepared by the above reaction if 0.850 g of CoI 2, 0.330 g of Cu, and 0.985 g of CO are mixed? (b) How many grams of dicobalt octacarbonyl may be prepared by reacting 36.7250 g of CoI 2 with an excess of the other reactants? 3.132 One reaction that leads to the tarnishing of silverware is: 4 Ag + 2 H2S + O2 2 Ag2S + 2 H2O A chemist, who is investigating this reaction mixes 1.6310 g of silver (Ag), 0.2560 g of hydrogen sulfide (H2S), and 0.1215 g of oxygen (O2) are mixed. How many grams of silver sulfide (Ag2S) will form? 3.133 Nickel tetracarbonyl is one of the most toxic substances known. The recommended maximum concentration in air is 29 mg/m3. At this maximum concentration, how many grams of nickel tetracarbonyl would be in a room that measures 25.0 ft × 15.5 ft × 8.0 ft? 3.134 Rubies and sapphires are aluminum oxide with different colors induced by various impurities such as chromium and manganese. The density of pure aluminum oxide is 3.96 g/cm3. How many aluminum atoms are present in a 3.50 cm3 sample of aluminum oxide? 3.135 One industrial method for the production of white phosphorus reaction of the ore apatite (Ca 5(PO4)3OH) in the presence of carbon and sand (mostly SiO 2) at very high temperatures. The phosphorus distills away. The unbalanced equation for the reaction is: Ca5(PO4)3OH (s) + C(s) + SiO2(s) P4(g) + CO(g) + CaSiO3(l) + H2O(g) (unbalanced) (a) Balance the above equation. (b) How many kilograms of apatite are necessary to prepare a metric ton (1000.0 kg) of P4? (c) How many kilograms of apatite are necessary to prepare a metric ton of P 4 if the percent yield is only 85.2%? (d) Determine the theoretical yield of P4 from the reaction of 115 kg of apatite, 19.1 kg of carbon, and 131 kg of sand. (e) What is the percent yield in part (d) if only 11.0 kg of P 4 formed? Chapter 4 4.1 A solution forms when sugar dissolves in water. What is the solute and what is the solvent? Is this an aqueous solution? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 27 4.2 (a) Give an example of a solution that is an aqueous solution. (b) Give an example of a solution that is not an aqueous solution. 4.3 Define the terms (a) unsaturated solution, (b) saturated solution, and (c) supersaturated solution. 4.4 Which of the following types of solutions may spontaneously form a precipitate: an unsaturated solution, a saturated solution, or a supersaturated solution? 4.2 Corresponds to BLBMWS Section 4.5 4.6 Define molarity. 4.7 What information do you need to prepare a 1 M solution? 4.8 When performing a dilution, which of the following increases: moles of solute, volume of solution, concentration of solute? 4.9 In the CRC Handbook of Chemistry and Physics, we find that 23.8 g of potassium chloride will dissolve in 100 cm3 of water at 20°C. What additional information is necessary to determine the molarity of the solution? 4.10 (a) What is the molarity of a solution containing 0.0335 mol of potassium dichromate (K 2Cr2O7) in 450.0 mL of solution? (b) Determine the number of milliliters of a 1.05 M calcium bromide (CaBr 2) solution required to supply 0.500 mol of solute. (c) Calculate the number of moles of sodium chloride (NaCl) in 125 mL of a 2.75 M solution of sodium chloride. (d) How many milliliters of a 0.100 M iron(III) chloride (FeCl 3) solution are required to supply 0.575 mol of chloride ion (Cl–)? 4.11 (a) What is the molarity of a solution containing 0.0435 mol of sodium hydrogen carbonate (NaHCO 3) in 375.0 mL of solution? (b) Determine the number of milliliters of a 2.05 M ethanol (C 2H5OH) solution required to supply 1.000 mol of solute. (c) Calculate the number of moles of ammonium hydrogen phosphate ((NH 4)2HPO4) in 225 mL of a 0.750 M solution of ammonium hydrogen phosphate. (d) How many milliliters of a 0.250 M iron(II) chloride (FeCl2) solution are required to supply 0.100 mol of chloride ion (Cl –)? (a) 0.116 M (b) 488 mL (c) 0.169 mole (d) 2.00 × 102 mL 4.12 How many grams of solute are required to make each of the following solutions? (a) 0.250 L of 0.250 M nitric acid (HNO3) (b) 175 mL of 0.500 M sulfuric acid (H2SO4) (c) 100.0 mL of 1.25 M phosphoric acid (H 3PO4) (d) 250. mL of 1.55 × 10–6 M hydrofluoric acid (HF) (e) 1250 mL of 10.0 M perchloric acid (HClO 4) 4.13 How many grams of solute are required to make each of the following solutions? (a) 0.500 L of 0.125 M chloric acid (HClO3) (b) 275 mL of 1.50 M periodic acid (HIO 4) (c) 1000.0 mL of 2.25 M acetic acid (HC2H3O2). (d) 500.0 mL of 2.50 × 10–5 M hydrochloric acid (HCl) (e) 1750 mL of 10.0 M nitric acid (HNO 3) 4.14 You have a bottle of pure magnesium chloride (MgCl2), and you need to prepare 250.0 mL of a 0.100 M solution of magnesium chloride. Calculate how much solute you would need, and tell how you would make the solution. 4.15 You have 2.00 L of a 3.50 M lithium chloride (LiCl) solution. Make the appropriate calculations and outline the steps you would take to prepare 1.00 L of 0.150 M lithium chloride. 4.16 Concentrated sulfuric acid is supplied in 4 L bottles of 18.0 M H 2SO4. Outline the steps you would take and show the necessary calculations to prepare 500.0 mL of 3.0 M sulfuric acid. 4.17 Ethylene glycol (C2H4(OH)2) is used in many antifreezes. At 20°C, the density of ethylene glycol is 1.1202 g/mL. Determine the molarity of an ethylene glycol solution made by adding 125.00 mL of this compound (at 20°C) to a container and adding sufficient water to produce a solution with a volume of 500.00 mL. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 28 4.18 Calculate the final molarity of acid in each of the following solutions. (a) 0.125 L of 1.50 M acetic acid (HC2H3O2) mixed with sufficient water to prepare 1.00 L of solution (b) 475 mL of 5.00 M nitric acid (HNO 3) added to 725 mL of water (assume the volumes are additive) (c) 5.33 g of dinitrogen pentoxide added to sufficient water to prepare 750.0 mL of solution 4.19 Commercial solutions of concentrated ammonia are 14.8 M. A chemist needs 750 mL of 3.00 M ammonia. Showing the necessary calculations, describe how this solution may be prepared from concentrated ammonia. Measure 152 mL of NH3 solution and dilute to 750 mL. 4.3 Corresponds to BLBMWS Section 4.1 4.20 (a) What is a cation? (b) What is an anion? 4.21 What is the difference between a nonelectrolyte and an electrolyte? Give an example of each. 4.22 What is the difference between a strong electrolyte and a weak electrolyte? Give an example of each. 4.23 What is the difference between a strong acid and a weak acid? Give an example of each. 4.24 Which of the following is an example of a strong base solution: 10.0 M NH3 or 0.001 M NaOH? Why? 4.25 What does the symbol "" means in a chemical equation? 4.26 Each of the following substances will dissolve, to a greater or lesser extent, in water. Predict which are nonelectrolytes and which are electrolytes. Then predict which of the electrolytes are strong or weak. (a) potassium chloride (KCl, a salt substitute) (b) sucrose (C12H22O11, table sugar) (c) acetic acid (HC2H3O2, in vinegar) (d) hydrochloric acid (HCl, stomach acid) (e) sodium bicarbonate (NaHCO3, baking soda) (f) ethylene glycol (C2H4(OH)2, in antifreeze) (g) ammonia (NH3, in some household cleaners) (h) isopropyl alcohol (CH 3CHOHCH3, rubbing alcohol) (i) copper(II) sulfate (CuSO4, used as an algaecide) (j) oxalic acid (H2C2O4, in some rust removers) 4.27 The following substances are soluble in water. Predict which behave as nonelectrolytes and which behave as electrolytes. If the substance is an electrolyte, predict whether it is a strong or weak electrolyte. (a) sulfuric acid (H2SO4, battery acid) (b) citric acid (H3C6H5O7, in citrus fruit) (c) ethanol (C2H5OH, grain alcohol) (d) magnesium sulfate heptahydrate (MgSO4•7H2O, Epsom salts) (e) fructose (C6H12O6, fruit sugar) (f) sodium hypochlorite (NaOCl, a component of some bleaches) (g) potassium iodide (KI, added to table salt to prevent goiter) (h) ascorbic acid (C6H8O6, vitamin C) (i) sodium stearate (NaC18H35O2, in some soaps) (j) sodium hydroxide (NaOH, lye, a component of some drain cleaners) 4.31 Acetic acid, structural formula pictured below, is a weak monoprotic acid. (A monoprotic acid has only one acidic hydrogen atom.) Examine the structure of acetic acid and predict which of the four hydrogen atoms in acetic acid is the only acidic hydrogen atom. H H O C C O H H 4.4 Corresponds to BLBMWS Section 4.2 4.32 (a) To which ions does the first solubility rule apply? (b) To which ions does the second solubility rule apply? (c) To which ions does the third solubility rule apply? 4.33 (a) List the exceptions to the first solubility rule. (b) List the exceptions to the second solubility rule. (c) List the exceptions to the third solubility rule. 4.34 What substances not covered by the solubility rules may be soluble in water? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 29 4.35 Predict which of the following are soluble in water. (a) potassium nitrate (KNO 3) (b) silver bromide (AgBr) (c) iron(III) phosphate (FePO4) (d) barium hydroxide (Ba(OH)2) (e) zinc hydroxide (Zn(OH)2) (f) aluminum sulfate (Al2(SO4)3) (g) lead(II) iodide (PbI2) (h) ammonium oxalate ((NH4)2C2O4) (i) sodium permanganate (NaMnO4) (j) mercury(I) chloride (Hg2Cl2) 4.36 Predict which of the following are soluble in water. (a) ammonium fluoride (b) strontium phosphate (c) zinc chromate (d) potassium phosphate (e) magnesium hydroxide (f) silver nitrate (g) barium acetate (h) calcium chloride (i) aluminum oxide (j) cesium hydroxide 4.5 Corresponds to BLBMWS Section 4.3 4.37 Define a neutralization reaction and give an example. 4.38 Which, if any, of the following would not be a neutralization reaction? (a) a strong acid with a strong base (b) a strong acid with a weak base (c) a weak acid with a strong base (d) a weak acid with a weak base 4.39 How does the existence of acidic oxides and basic oxides illustrate the difference between metals and nonmetals? 4.40 Label each of the following as an acid or a base; then label them as weak or strong. (a) HCl (b) HClO 2 (c) Ba(OH)2 (d) HC2H3O2 (e) NH3 4.41 Label each of the following as an acid or a base; then label them as weak or strong. (a) LiOH (b) HCN (c) H2SO4 (d) H2C2O4 (e) RbOH 4.42 Write balanced chemical equations showing how each of the following basic oxides forms a base when added to water. (a) K2O (b) CaO (c) lithium oxide (d) barium oxide 4.43 Write balanced chemical equations showing how each of the following acidic oxides forms a strong acid when added to water. (a) SO3 (b) Cl2O5 (c) dinitrogen pentoxide (d) dichlorine heptaoxide 4.44 Determine the products for each of the following reactions; then balance the chemical equations: (a) NaOH(aq) + HNO3(aq) (b) H2SO4(aq) + LiOH(aq) (c) Sr(OH)2(aq) + H2C2O4(aq) (d) H3PO4(aq) + Cu(OH)2(s) (e) Fe(OH)3(s) + H2SeO4(aq) 4.45 The following depictions show two compounds containing an OH group. One of these compounds, sodium hydroxide, is a strong base, while the other compound, ethyl alcohol, is not a base. Examine the two depictions to find a reason for this difference in behavior. Na+ OH- sodium hydroxide H H H C C H H O H ethyl alcohol 4.6 Corresponds to BLBMWS Section 4.4 4.46 Why is it not possible to have an oxidation without a reduction? 4.47 If copper undergoes oxidation in a reaction, is copper the oxidizing agent or the reducing agent? 4.48 Why are certain metals placed near the top of the activity series whereas certain other metals are placed near the bottom? 4.49 What is the relationship between the electrons lost and the electrons gained in a redox reaction? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 30 4.50 Calculate the oxidation number for each element in each of the following: (a) SO 3 (b) SO32– (c) HC2H3O2 (d) FeF63– (e) Hg2Br2 (f) NO2+ (g) K2CrO4 (h) H2CO (i) CHO2– (j) Fe3O4 4.51 Based on their positions on the periodic table, list the maximum and minimum oxidation numbers expected for each of the following elements: (a) As (b) S (c) Cr (d) Ba (e) Cu (a) As +5 to –3 (b) S +6 to –2 (c) Cr +6 to 0 (d) Ba +2 to 0 (e) Cu +2 to 0 4.52 Give the name or the formula, as appropriate, for each of the following: (a) PbO 2 (b) Cu2O (c) Mn2O7 (d) CrO (e) TiO2 (f) tungsten(VI) oxide (g) copper(II) sulfide (h) nickel(II) bromide (i) gold(III) oxide (j) silver(II) fluoride 4.53 Give the name or the formula, as appropriate, for each of the following: (a) Cu(NO 3)2 (b) TiCl3 (c) NiCO3 (d) Ag2O3 (e) Fe3(AsO4)2 (f) tin(II) fluoride (g) molybdenum(IV) oxide (h) chromium(III) hydroxide (i) manganese(II) sulfate (j) cobalt(II) phosphate 4.54 Identify which elements in each of the following reactions change oxidation states. (a) 3 Cu(s) + 8 HNO3(aq) 3 Cu(NO3)2(aq) + 2 NO(g) + 4 H2O(l) (b) 2 Na2O2(s) + 2 H2O(l) 4 NaOH(aq) + O2(g) (c) 2 KMnO4(aq) + 5 H2C2O4(aq) + 3 H2SO4(aq) K2SO4(aq) + 2 MnSO4(aq) + 10 CO2(g) + 8 H2O(l) (d) CO(g) + 2 H2(g) CH3OH(g) (e) 3 Br2(aq) + 6 KOH(aq) KBrO3(aq) + 5 KBr(aq) + 3 H2O(l) 4.7 Corresponds to BLBMWS Section 4.1-4.2 4.55 Define a molecular equation, a total ionic equation, and a net ionic equation, and give an example of each. 4.56 Define a spectator ion. 4.57 List the types of substances that dissociate in a total ionic equation. Give one example of each type. 4.58 How do the solubility rules help you write a total ionic equation? 4.59 Convert the following balanced chemical reactions to net ionic equations and list the spectator ions, if any. (a) 2 Al(s) + 6 HBr(aq) 2 AlBr3(aq) + 3 H2(g) (b) Mg(s) + 2 AgNO3(aq) Mg(NO3)2(aq) + 2 Ag(s) (c) 3 Fe(s) + Au2(SO4)3(aq) 3 FeSO4(aq) + 2 Au(s) (d) 2 Cs(s) + 2 H2O(l) 2 CsOH(aq) + H2(g) (e) Zn(s) + PdCl2(aq) ZnCl2(aq) + Pd(s) 4.60 Convert the following to net ionic equations and list any spectator ions. (a) Ba(NO3)2(aq) + (NH4)2SO4(aq) 2 NH4NO3(aq) + BaSO4(s) (b) PbO(s) + H2SO4(aq) PbSO4(s) + H2O(l) (c) 2 RbOH(aq) + Mn(NO2)2(aq) Mn(OH)2(s) + 2 RbNO2(aq) (d) 2 Al(s) + 6 HBr(aq) 2 AlBr3(aq) + 3 H2(g) (e) 2 H3AsO4(aq) + 3 CoI2(aq) 6 HI(aq) + Co3(AsO4)2(s) (a) Ba2+(aq) + SO42–(aq) BaSO4(s) NH4+ and NO3– + 2– (b) PbO(s) + 2 H (aq) + SO4 (aq) PbSO4(s) + H2O(l) None (c) 2 OH–(aq) + Mn2+(aq) Mn(OH)2(s) Rb+ and NO2– + 3+ (d) 2 Al(s) + 6 H (aq) 2 Al (aq) + 3 H2(g) Br– (e) 2 H3AsO4(aq) + 3 Co2+(aq) 6 H+(aq) + Co3(AsO4)2(s) I– 4.61 Balance the following reactions, convert to net ionic equations, and list the spectator ion(s), if any. (a) LiCl(aq) + AgNO3(aq) LiNO3(aq) + AgCl(s) (b) K2CO3(aq) + CuCl2(aq) KCl(aq) + CuCO3(s) (c) FeS(s) + HCl(aq) FeCl2(aq) + H2S(g) (d) (NH4)3AsO4(aq) + Ca(MnO4)2(aq) Ca3(AsO4)2(s) + NH4MnO4(aq) (e) BaCO3(s) + H3PO4(aq) Ba3(PO4)2(s) + CO2(g) + H2O(l) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 31 4.62 Complete and balance the following equations using the activity series. Write molecular and net ionic equations for each. (a) Copper metal is added to an aqueous silver nitrate solution. (b) Zinc metal is added to an aqueous sulfuric acid solution. (c) Iron metal is added to an aqueous aluminum nitrate solution. (d) Tin metal is added to an aqueous copper(II) sulfate solution. (e) Potassium metal is added to water. (a) Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s) Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) (b) Zn(s) + H2SO4(aq) ZnSO4(aq) + H2(g) Zn(s) + 2 H+(aq) Zn2+(aq) + H2(g) (c) Fe(s) + Al(NO3)3(aq) No Reaction Fe(s) + Al3+(aq) No Reaction (d) Sn(s) + CuSO4(aq) SnSO4(aq) + Cu(s) Sn(s) + Cu2+(aq) Sn2+(aq) + Cu(s) (e) 2 K(s) + 2 H2O(l) 2 KOH(aq) + H2(g) 2 K(s) + 2 H2O(l) 2 K+(aq) + 2 OH–(aq) + H2(g) 4.63 Write balanced equations for the following reactions. Write both molecular and net ionic equations for each. (a) Zinc reacts with chloric acid. (b) Iron reacts with hydrochloric acid. (c) Magnesium reacts with acetic acid. (d) Nickel reacts with hydrosulfuric acid. (e) Sodium reacts with water 4.64 Write balanced equations for the following reactions. Write both molecular and net ionic equations for each. (a) Lead reacts with nitric acid. (b) Calcium reacts with oxalic acid. (c) Cobalt reacts with sulfuric acid. (d) Chromium reacts with perchloric acid. (e) Barium reacts with water 4.65 Complete and balance the following equations using the activity series. Write both molecular and net ionic equations. (a) Zn(s) + CuSO4(aq) (b) Mg(s) + ZnSO4(aq) (c) H2(g) + AuCl3(aq) (d) Fe(s) + HCl(aq) (e) Cu(s) + AgNO3(aq) (f) Au(s) + Al2(SO4)3(aq) (g) Ba(s) + H2O(l) (h) Sn(s) + Pb(C2H3O2)2(aq) (i) Hg(l) + AgClO3(aq) (j) Cr(s) + NiI2(aq) 4.66 Complete and balance the following equations using the Activity Series. Write both molecular and net ionic equations. (a) Mg(s) + CuSO4(aq) (b) Mg(s) + Ag2SO4(aq) (c) H2(g) + AgCl(s) (d) Ni(s) + HBr(aq) (e) Cu(s) + Hg(NO3)2(aq) (f) Hg(l) + Al2(SO4)3(aq) (g) Ca(s) + H2O(l) (h) Zn(s) + Pb(C2H3O2)2(aq) (i) Fe(s) + H2S(aq) (j) Cr(s) + CoI2(aq) 4.8 Corresponds to BLBMWS Section 3.2 4.69 What is a metathesis reaction? 4.70 Write a balanced chemical equation for a reaction that results in the formation of a gas from substances in aqueous solution. 4.71 Balance the following reactions, convert them to net ionic equations, and list the spectator ions, if any. (a) KOH(aq) + (NH4)2SO4(aq) K2SO4(aq) + H2O(l) + NH3(g) (b) H3PO4(aq) + CaSO3(s) Ca3(PO4)2(s) + H2O(l) + SO2(g) (c) FeS(s) + HCl(aq) FeCl2(aq) + H2S(g) (d) HCl(aq) + CaCO3(s) CaCl2(aq) + H2O(l) + CO2(g) (e) NH4NO3(aq) + K2CO3(aq) KNO3(aq) + H2O(l) + NH3(g) + CO2(g) 4.72 Complete the equations for the reactions occurring when the following substances are mixed. Write both molecular and net ionic equations for each. If there is no reaction, label the answer as NR. (a) K2SO4(aq) with Pb(NO3)2(aq) (b) Al2(SO4)3(aq) with Na2CO3(aq) (c) HC2H3O2(aq) with Zn(OH)2(s) (d) Hg2(NO3)2(aq) with HCl(aq) (e) CrCl3(aq) with Na2CrO4(aq) (f) (NH4)2C2O4(aq) with K2SO4(aq) (g) ZnS(s) with HNO3(aq) (h) FeCl2(aq) with CsOH(aq) (i) H2SO4(aq) with MgCO3(s) (j) AgNO3(aq) with NaCl(aq) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 32 4.73 Complete the molecular equations for any reactions resulting when the following substances are mixed, then write the net ionic equations for each. If there is no reaction, label the answer as NR. (a) Al(NO3)3(aq) with Na3PO4(aq) (b) KOH(aq) with H2CO3(aq) (c) Pb(NO3)2(aq) with HBr(aq) (d) Ba(OH)2(aq) with Fe(NO3)3(aq) (e) AgClO4(aq) with SrBr2(aq) (f) NH4NO3(aq) with KOH(aq) (g) NaNO3(aq) with CaCl2(aq) (h) NiBr2(aq) with H2S(aq) (i) HNO2(aq) with Mn(OH)2(s) (j) Ba(NO3)2(aq) with (NH4)2SO4(aq) 4.74 Balance the following chemical equations by placing appropriate coefficients in the blanks. Then classify the type of reaction as decomposition, combination, combustion, or “other.” (a) ___XeO3(s) ___Xe(g) + ___O2(g) (b) ___Pt(s) + ___F2(g) ___PtF6(s) (c) ___C10H22(l) + ___O2(g) ___CO2(g) + ___H2O(g) (d) ___P4(s) + ___O2(g) ___P4O10(s) (e) ___Ca(OH)2(aq) + ___HCl(aq) ___CaCl2(aq) + ___H2O(l) 4.75 Balance the following chemical equations by placing appropriate coefficients in the blanks. Then classify the type of reaction as decomposition, combination, combustion, or “other.” (a) ___Mg(s) + ___O2(g) ___MgO(s) (b) ___Mg(s) + ___N2(g) ___Mg3N2(s) (c) ___SO2(g) + ___H2O(l) ___H2SO3(aq) (d) ___HgO(s) ___Hg(l) + ___O2(g) (e) ___K(s) + ___H2O(l) ___KOH(aq) + ___H2(g) 4.76 Write balanced molecular and net ionic equations for the reaction of aqueous sulfuric acid, H2SO4, with each of the following: (a) a sodium hydroxide (NaOH) solution (b) solid aluminum hydroxide (Al(OH) 3) (c) a barium chloride (BaCl2) solution (d) a potassium carbonate (K2CO3) solution (e) a rubidium fluoride (RbF) solution. 4.77 Write balanced molecular and net ionic equations for the reaction of aqueous phosphoric acid, H 3PO4, with each of the following: (a) a potassium hydroxide (KOH) solution (b) solid aluminum hydroxide (Al(OH) 3) (c) a calcium chloride (CaCl2) solution (d) a sodium carbonate (Na2CO3) solution (e) a cesium fluoride (CsF) solution. 4.9 Corresponds to BLBMWS Section 4.6 4.78 (a)How many grams of solid form when 50.00 mL of 2.500 M CuCl 2 solution reacts as follows with an excess of Rb3AsO3 solution? (b) What is the percent yield if only 17.95 grams of solid form? (c) How many grams of solid will form by reacting 37.50 mL of 0.1750 M CuCl 2 solution with 25.00 mL of 0.1500 M Rb 3AsO3 solution? 3 CuCl2(aq) + 2 Rb3AsO3(aq) Cu3(AsO3)2(s) + 6 RbCl(aq) (a) 18.19 g Cu3(AsO3)2 (b) 98.70 % (c) 0.3637 g Cu3(AsO3)2 4.79 How many milliliters of 0.2300 M Na2C2O4 solution are required to react as follows with 17.00 g of ThCl 4? ThCl4(aq) + 2 Na2C2O4(aq) Th(C2O4)2(s) + 4 NaCl(aq) 4.80 How many grams of NaCl are produced when 25.00 mL of a 0.1000 M NiCl 2 solution react as follows with an excess of Na2CrO4 solution? NiCl2(aq) + Na2CrO4(aq) NiCrO4(s) + 2 NaCl(aq) 4.81 How many milliliters of a 0.1400 M K2S2O3 solution are required to produce 1.000 g of Pu(S2O3)2 in the reaction PuCl4(aq) + 2 K2S2O3(aq) Pu(S2O3)2(s) + 4 KCl(aq) 4.82 How many milliliters of a 1.125 M K2C2O4 solution are required to produce 5.000 g of U(C2O4)2 in the reaction UCl4(aq) + 2 K2C2O4(aq) U(C2O4)2(s) + 4 KCl(aq) 4.83 The following reaction produced 2.850 g of NaCl. If 100.00 mL of AuCl 3 solution was used, what was the concentration of the AuCl3 solution? AuCl3(aq) + Na3PO3(aq) AuPO3(s) + 3 NaCl(aq) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 33 4.84 What is the concentration of base when 350.0 mL of 0.2010 M sulfuric acid reacts with 0.5000 L of sodium hydroxide? 0.2814 M NaOH 4.85 How many milliliters of 0.1750 M Ti(SO4)2 solution are required to react as follows with 25.00 mL of a 0.1750 M Na2SO3 solution? Ti(SO4)2(aq) + 2 Na2SO3(aq) Ti(SO3)2(s) + 2 Na2SO4(aq) 4.86 (a) How many grams of LiCl are produced when 25.00 mL of 1.000 M VCl 3 solution react as follows with an excess of Li3AsO4? (b) How many grams of LiCl will form when 37.50 mL of 0.7500 M VCl3 solution react with 25.00 mL of a 0.1000 M Li3AsO4 solution? VCl3(aq) + Li3AsO4(aq) VAsO4(s) + 3 LiCl(aq) 4.87 What is the concentration of base when 350.0 mL of 0.2010 M sulfuric acid reacts with 0.1500 L of lithium hydroxide? 4.88 What is the concentration of acid when 45.35 mL of 0.01000 M calcium hydroxide reacts with 50.00 mL of phosphoric acid? 4.89 How many grams of MoO3 could we dissolve by the following reaction using 100.00 mL of 0.1000M HCl? MoO3(s) + 6 HCl(aq) MoCl6(aq) + 3 H2O(l) 4.90 How many milliliters of 0.1550 M magnesium chloride are necessary to precipitate the silver ion completely from a solution made by dissolving 2.500 g of silver nitrate in 500.0 mL of water? 4.91 (a) After 25.00 mL of a 0.1200 M CoCl2 solution were reacted as shown below with an excess of a 0.1800 M Na2CO3 solution, the resulting mixture was diluted to 250.00 mL. What was the final concentration of NaCl? (b) Titration of the final solution determined the concentration of NaCl to be 0.0220 M. What was the percent yield of the reaction? CoCl2(aq) + Na2CO3(aq) CoCO3(s) + 2 NaCl(aq) 4.92 The following reaction generates HCl. (a) How many grams of HCl could we produce by reacting 50.00 mL of a 0.2000 M CuCl2 solution with an excess of a 0.3000 M H2CrO4 solution? (b) If only 0.65 g of HCl formed, what was the percent yield? CuCl2(aq) + H2CrO4(aq) CuCrO4(s) + 2 HCl(aq) 4.93 The following reaction produced 2.875 g of KNO3. (a) How many milliliters of a 0.1000 M K2CO3 solution were required to do this? (b) How many grams of CO2(g) will escape? K2CO3(aq) + 2 HNO3(aq) 2 KNO3(aq) + CO2(g) + H2O(l) 4.10 Summary 4.94 Someone gives you two unlabeled beakers. You know that one beaker contains a barium nitrate solution and one beaker contains a magnesium nitrate solution. Since solutions containing barium ions are toxic, it is important to know which of the beakers contains barium ion. Which of the following substances could be used to distinguish between barium ions and magnesium ions: NaNO3, CaCl2, (NH4)2SO4, KBr, or HBr? Write a balanced chemical equation for any reactions that occur 4.95 Even though magnesium hydroxide [Mg(OH)2] is classified as an insoluble substance, a very small amount of the solid will dissolve in water. A sample of magnesium hydroxide is added to a liter of water and allowed to stand overnight. The next day, 500.0 mL of solution is carefully withdrawn and found to require 45.25 mL of 3.6 x 10 –3 M hydrochloric acid (HCl) for neutralization. (a) Write balanced molecular and net ionic equations for the neutralization reaction. (b) Calculate the molarity of magnesium hydroxide. (c) How many grams of magnesium hydroxide dissolved in the 500.0 mL solution? (d) How many milliliters of water are required to dissolve 1.0 g of magnesium hydroxide? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 34 4.96 Toxic mercury ions may be removed from a solution by precipitating mercury(II) sulfide (HgS). A solution of sodium sulfide (Na2S) is made by dissolving 50.00 g in sufficient water to prepare 0.7500 L of solution. How many milliliters of the sodium sulfide solution are necessary to precipitate the mercury ions from 10.00 L of a solution that is 1.58 x 10–3 M Hg2+? 18.5 mL 4.97 (a) Determine how many milliliters of a 1.25 x 10 –3 M calcium hydroxide [Ca(OH)2] solution are needed to completely react with 100.0 mL of 0.1000 M chloric acid (HClO 3). (b) What volume of 0.2500 M perchloric acid (HClO4) solution is needed to dissolve 0.1000 g of iron(II) hydroxide [Fe(OH) 2]? (c) Calculate the milliliters of 0.1255 M hydrochloric acid (HCl) required to precipitate all the Pb 2+ ions from a solution made by dissolving 0.5000 g of lead(II) acetate [Pb(C2H3O2)2] in 1.000 L of water. (d) What is the molarity of a chlorous acid (HClO 2) solution if 45.25 mL of 0.1025 M sodium hydroxide (NaOH) solution are required to neutralize 25.00 mL of a solution of the acid? (e) How many grams of hypochlorous acid (HOCl) are present in a solution that requires 40.35 mL of 0.09825 M barium hydroxide [Ba(OH) 2] solution to neutralize? (a) 4.000 × 103 mL (b) 0.008903 L (c) 24.50 mL (d) 0.1855 M (e) 0.4159 g 4.98 Vinegar is normally 5–6% acetic acid. A 5.00-g sample of vinegar is titrated with 0.2243 M sodium hydroxide (NaOH) solution. If the titration requires 44.32 mL of base to neutralize the vinegar, in the following reaction, what is the percentage of acetic acid in the vinegar? HC2H3O2(aq) + NaOH(aq) H2O(l) + NaC2H3O2(aq) 4.99 Citric acid (H3C6H5O7), found in citrus fruits, such as lemons, has three hydrogens that may react with bases. A 25.00-mL solution of this acid is titrated with 0.01837 M sodium hydroxide (NaOH) solution. The titration requires 47.23 mL of base. (a) Write a balanced molecular equation for the reaction of citric acid with sodium hydroxide. (b) What is the molarity of the citric acid solution? 4.100 Iron forms two chlorides: iron(II) chloride (FeCl2) and iron(III) chloride (FeCl3). Both are soluble in water. A solution is prepared by dissolving 0.6825 g of one of these chlorides in 250 mL of water. A solution of silver nitrate (AgNO3) is added to the iron chloride solution, and 1.809 g of silver chloride (AgCl) precipitates. (a) Determine the mass percent of chloride in iron(II) chloride and in iron(III) chloride. (b) Based on the grams of silver chloride precipitated, what was the mass percentage of chloride in the sample dissolved in water? (c) Write a balanced chemical equation for the reaction of silver nitrate with the iron chloride determined to be present in the sample. 4.11 Summary 4.101 (a) Calculate the molarity of nitrate ion (NO3–) in a solution made by mixing 500.0 mL of 0.2500 M sodium nitrate (NaNO3) with 750.0 mL of 0.1250 M potassium nitrate (KNO 3). (b) Calculate the molarity of nitrate ion in a solution made by mixing 75.0 mL of 0.2500 M sodium nitrate with 75.0 mL of 0.3750 M calcium nitrate [Ca(NO3)2]. Assume the volumes are additive in both cases. 4.102 An environmental chemist dissolves a sample of sulfuric acid (H 2SO4) weighing 2.75 g in 500.0 mL of a 0.125 M sulfuric acid solution. The volume is then adjusted to 750.0 mL. What is the final concentration of sulfuric acid? 4.103 Give the formula and the name of the salt produced in each of the following neutralization reactions. (a) aqueous potassium hydroxide reacts with aqueous nitric acid (b) solid magnesium hydroxide dissolves in aqueous hydrochloric acid (c) aqueous acetic acid reacts with aqueous barium hydroxide (d) aqueous arsenic acid (H 3AsO4) reacts with solid zinc hydroxide (e) gaseous ammonia dissolves in aqueous phosphoric acid (a) KNO3 Potassium Nitrate (b) MgCl2 Magnesium Chloride (c) Ba(C2H3O2)2 Barium Acetate (d) Zn3(AsO4)2 Zinc Arsenate (e) (NH4)3PO4 Ammonium Phosphate 4.104 Zinc metal will reduce V3+(aq) ions to V2+(aq) ions. The reaction must be run in the absence of air, as oxygen in the air will react with the V2+(aq) ions. (a) Write the balanced net ionic equation for this process. (b) Why would this reaction be inappropriate as a means of placing vanadium in the activity series? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 35 4.105 An activity series may be constructed for the halogens. The following reactions occur for some of the halogens: Cl2(g) + 2 KBr(aq) Br2(l) + 2 KCl(aq) Cl2(g) + 2 KI(aq) I2(s) + 2 KCl(aq) Br2(l) + 2 KI(aq) I2(s) + 2 KBr(aq) All other combinations of these three halogens and their ions show no reaction. (a) Convert the three reactions to net ionic equations. (b) Place these three halogens into an activity series beginning with the most reactive. (c) Compare your activity series to the arrangement of these three halogens on the periodic table. (d) Where would you expect fluorine to appear in your activity series? (e) Using your activity series, predict what will occur when each of the following are mixed: Cl2(g) + NaBr(aq) Br2(l) + KCl(aq) I2(s) + LiCl(aq) Br2(l) + LiI(aq) F2(g) + RbCl(s) 4.106 Determine the molarity of each solute particle in the following solutions. (a) 0.250 M hydrochloric acid (HCl) (b) 0.500 M sodium fluoride (NaF) (c) 0.125 M strontium bromide (SrBr2) (d) 5.00 M ethyl alcohol (C2H5OH) (e) a solution prepared by mixing 125 mL of 0.100 M potassium chloride (KCl) solution with 275 mL of a 0.0500 M calcium chloride (CaCl2) solution 4.107 Rank the following in order of decreasing amount of ammonium ion present. (a) 25.0 mL of 0.100 M ammonium chloride (NH4Cl) (b) 45.0 mL of 0.0750 M ammonium nitrate (NH 4NO3) (c) 25.0 mL of 0.100 M ammonium sulfate ((NH4)2SO4) 4.108 Determine the formulas for all the reactants and products for the following reactions. Then write balanced chemical equations for each reaction. (a) When solid magnesium is heated in the presence of nitrogen gas, solid magnesium nitride forms. (b) Solid aluminum metal reacts with a dilute aqueous solution of sulfuric acid to produce hydrogen gas and an aqueous solution of aluminum sulfate. (c) Liquid thionyl chloride (SOCl 2) reacts with water vapor to produce sulfur dioxide gas and hydrogen chloride gas. (d) Methane gas burns in oxygen gas to form gaseous carbon dioxide and water vapor. (e) Solid calcium hydroxide reacts with an aqueous solution of phosphoric acid to form solid calcium phosphate and liquid water. 4.109 (a) How many grams of solid could you produce by the following reaction if 100.00 mL of a 0.2000 M CoCl 2 solution was reacted with an excess of a 0.5000 M K3AsO4 solution? 3 CoCl2(aq) + 2 K3AsO4(aq) 6 KCl(aq) + Co3(AsO4)2(s) (b) How many grams of solid could you produce by the following reaction if 100.00 mL of a 0.2000 M CoCl2 solution was reacted with 25.00 mL of a 0.5000 M K3AsO4 solution? (a) 3.031 g Co3(AsO4)2 (b) 2.841 g Co3(AsO4)2 4.110 (a) Calculate the volume of 0.1245 M sodium hydroxide (NaOH) solution needed to completely react with 25.00 mL of 0.1500 M hydrochloric acid (HCl) solution. (b) Calculate the volume of 0.1621 M sulfuric acid (H2SO4) solution required to neutralize 50.00 mL of 0.4521 M potassium hydroxide (KOH) solution. (c) How many milliliters of 1.25 M nitric acid (HNO3) solution are required to react with 2.50 g of calcium hydroxide (Ca(OH)2)? (d) How many milliliters of 0.1250 M magnesium chloride (MgCl 2) are needed to precipitate all of the silver ion from a solution made by dissolving 1.00 g of silver nitrate (AgNO3) in 500.0 mL of water? (e) How many grams of sodium chromate (Na2CrO4) are in a solution if it reacted completely with 750.0 mL of a 0.7500 M barium nitrate (Ba(NO3)2) solution? 4.111 (a) How many grams of LiCl can the following reaction produce from 25.00 mL of 1.000 M VCl 3 solution with an excess of Li3AsO4? (b) How many grams of VAsO 4 form at the same time? (c) What is the percent yield if only 3.500 g of VAsO4 form? VCl3(aq) + Li3AsO4(aq) VAsO4(s) + 3 LiCl(aq) 4.112 How many milliliters of 0.2300 M Na2C2O4 solution are required to react with 17.00 g ThCl4? The reaction is ThCl4(aq) + 2 Na2C2O4(aq) Th(C2O4)2(s) + 4 NaCl(aq) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 36 4.113 The following reaction produces solid PtHPO4 and leaves KCl in solution. It is possible to recover the KCl by evaporation of the solution. (a) How many grams of KCl could a student produce by reacting 25.00 mL of a 0.1725 M PtCl2 solution and an excess of a 0.2200 M K2HPO4 solution? (b) What is the percent yield if the student isolates 0.4500 g of KCl? PtCl2(aq) + K2HPO4(aq) PtHPO4(s) + 2 KCl(aq) 4.114 Write balanced molecular and net ionic equations for each of the following combinations. (Assume that all reactions take place in aqueous solution.) (a) Nitric acid plus potassium acetate (b) Hydrochloric acid plus lithium thiocyanate (c) Rubidium oxalate plus barium bromide (d) Strontium hydroxide plus chloric acid (e) Sodium phosphate plus calcium chloride 4.115 Write balanced molecular and net ionic equations for each of the following combinations. (Assume that all reactions take place in aqueous solution.) (a) hydrochloric acid plus calcium acetate (b) sulfuric acid plus ammonium thiocyanate (c) sodium oxalate plus barium hydroxide (d) barium hydroxide plus perchloric acid (e) potassium phosphate plus strontium bromide 4.116 Which will contain more total moles of bromide ion: 45.0 mL of 0.53 M sodium bromide or 55.0 mL of 0.52 M calcium bromide? 4.117 Seawater contains an average of 65 mg Br– per kilogram of seawater. If the density of seawater is 1.025 g/mL, what is the molarity of bromide ion? 8.3 × 10–4 M 4.118 A 2.54-g sample of a pesticide was analyzed for arsenic. The arsenic was converted to AsO 43–, which was precipitated with Ag+ to produce solid Ag3AsO4. If 35.00 mL of 0.0955 M Ag+ solution was used to precipitate the solid, what was the mass percentage of arsenic in the pesticide? 4.119 Acetic acid is a weak acid capable of donating a hydrogen ion and thus becoming an acetate ion. We may write the formula of acetic acid in a number of ways. Two common ways are (I) HC 2H3O2 and (II) CH3COOH. The following figure shows the structural formula of acetic acid and the structural formula of the acetate ion. Using the structural formula as a guide, discuss an advantage for each of the two ways (I and II) of writing the formula of acetic acid. H H O C C O H H H O C C H H acetic acid acetate ion O- 4.120 The following reaction generates HCl. (a) How many grams of HCl could we produce by reacting 50.00 mL of a 0.2000 M ZnCl2 solution with an excess of a 0.3000 M H2CrO4 solution? (b) Titration of the generated HCl with 1.000 M NaOH required 13.70 mL of base. What was the percent yield of HCl? ZnCl2(aq) + H2CrO4(aq) ZnCrO4(s) + 2 HCl(aq) 4.121 The analysis of a 0.5000-g sample of a mixed metal oxide found manganese and iron in addition to oxygen. The sample was dissolved in excess sulfuric acid, neutralized, and the iron and manganese both reduced to the +2 ions. The sample was divided into two equal portions. The iron in one portion was then titrated with 43.27 mL of 0.2010 M potassium permanganate solution. The reaction was 5 Fe2+(aq) + MnO4–(aq) + 8 H+(aq) 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l) The second portion was titrated with 39.13 mL of 0.1660 M sodium hydroxide solution to precipitate a mixture of Fe(OH)2 and Mn(OH)2. (a) How many grams of iron were in the original sample? (b) How many grams of manganese were in the original sample? (c) How many grams of oxygen were in the original sample? (d) What was the empirical formula of the compound in the sample? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 37 4.122 The analysis of an unknown solid found iron, ammonium ions, sulfate ions, and water. The following titration reactions on separate 1.0000-g samples led to the results in the table below. (The titration of the ammonium ion required the removal of the iron first.) 5 Fe2+(aq) + MnO4–(aq) + 8 H+(aq) 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l) NH4+(aq) + OH–(aq) NH3(aq) + H2O(l) SO42–(aq) + Ba2+(aq) BaSO4(s) Concentration Volume required 0.01260 M KMnO4 40.37 mL 0.1335 M NaOH 38.29 mL 0.1200 M BaCl2 42.36 mL (a) How many grams of iron were in the sample? (b) How many grams of ammonium ions were in the sample? (c) How many grams of sulfate ion were in the sample? (d) How many grams of water were in the sample? (e) What was the empirical formula of the compound in the sample? Chapter 5 5.1 What is the difference between a gas and a vapor? 5.2 List the two condensed phases. 5.3 What are the postulates of kinetic molecular theory? 5.2 Corresponds to BLBMWS Sections 10.1-10.2 and 10.6 5.4 A 125-lb woman places her entire weight on one heel. If she is wearing high heels with an area at the heel’s end of 0.75 in2, what is the pressure under her heel in pascals? 5.5 A chemistry textbook weighing 4.53 lb rests on a desktop. The book is lying on its side, which is 10.25 in high and 8.12 in wide. What is the pressure under the book in pascals? 5.6 Make the following conversions: (a) 1.5 × 105 Pa to atmospheres (b) 852 torr to atmospheres (c) 1.95 kPa to atmospheres (d) 127 mmHg to torr (e) 827 torr to pascals. 5.7 Convert each of the following to torr. (a) 15.2 lb/in2 (b) 3.52 bar (c) 1.00 atm (d) 555 mmHg (e) 3.55 × 10 3 Pa 5.8 Determine the total pressure, in atmospheres, in a balloon that contains 0.950 atm nitrogen gas, 181 torr of oxygen gas, and 9 mmHg of argon. 5.9 A sample of air has a nitrogen partial pressure of 1072 torr, an oxygen partial pressure of 271 mmHg, and an argon partial pressure of 0.0179 atm. What is the total pressure of the sample in pascals? 5.10 Determine the mole fraction of each component in a gas mixture with a nitrogen partial pressure of 0.855 atm, an oxygen partial pressure of 0.220 atm, and a carbon dioxide partial pressure of 0.100 atm. 5.11 A gas mixture contains 655 torr of nitrogen, 245 torr of oxygen, and 25.2 torr of water vapor. Determine the mole fraction of each of the components of the mixture. 5.3 Corresponds to BLBMWS Section 10.3 5.14 Describe how placing a balloon in the freezer illustrates Charles' Law. 5.15 You place a small piece of dry ice (solid carbon dioxide) into a balloon and then seal the balloon. The balloon slowly expands, even though neither the temperature nor the pressure changes. Why does the volume of the balloon increase? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 38 5.17 (a) According to Boyle's law, pressure and volume are inversely proportional. Write a sentence or two explaining what "inversely proportional" means. (b) According to Charles's law, temperature and volume are proportional. Write a sentence or two explaining what "proportional" means. (c) Is the volume of a gas proportional or inversely proportional to the number of moles present? 5.18 Fill in the blank in each of the vertical columns below with either I (increases), D (decreases), or C (constant). The potential changes apply to a sample of gas: Condition 1 Condition 2 Condition 3 Condition 4 Condition 5 Volume constant __(b)___ constant constant increases Pressure increases constant increases __(d)___ __(e)___ Temperature constant decreases __(c)___ increases constant Moles _(a)____ constant constant constant constant 5.19 Fill in the blank in each of the vertical columns below with either I (increases), D (decreases), or C (constant). The potential changes apply to a sample of gas: Condition 1 Condition 2 Condition 3 Condition 4 Condition 5 Volume increases constant decreases __(d)___ constant Pressure constant decreases constant increases __(e)___ Temperature __(a)___ constant constant constant decreases Moles constant __(b)___ __(c)___ constant constant 5.4 Corresponds to BLBMWS Section 10.4 5.20 The ideal gas constant, R, has a value of 0.08206 L•atm/mol•K. What is the value of the ideal gas constant in ft3•psi/mol•K? 5.21 Determine the missing quantity in each row in the following table: P V n T (a) ________ 13.2 L 2.35 mol 22.5°C (b) 525.3 torr _______ 3.751 mol 27.85°C (c) 3.22 atm 1375 mL ____mol 315 K (d) 235 kPa 1652 mL 0.227 mol ____°C (a) 4.32 atm (b) 134.0 L (c) 0.171 mol (d) –67°C 5.22 Your body heats the air in your lungs to body temperature (37°C). If you exhale 1075 mL of air at a pressure of 755 mmHg, how many molecules of air did you exhale? 5.23 Steel cylinders are used to transport small quantities of oxygen gas. A particular cylinder has a volume of 50.0 L, and at 24.0°C contains oxygen gas under a pressure of 1225 lb/in2. (a) Assuming the oxygen is behaving ideally, how many grams of oxygen gas are present in the cylinder? (b) What would be the volume in milliliters of the gas at 27°C and 1.00 atm (1 atm = 14.70 lbs/in2)? 5.24 (a) What is the density (in grams per liter) of gaseous carbon dioxide (CO 2) at 0.893 atm and 47°C? (b) A sample of an unknown gas weighing 0.144 g has a volume of 275 mL at 755 mmHg and 97°C. What is the molar mass of the gas? (a) 1.50 g/L (b) 16.0 g/mol 5.25 A common method for determining the molar mass of a volatile liquid is the Dumas method. A sample of a volatile liquid is placed in a flask and vaporized in a boiling water bath. The flask is then removed from the bath and cooled until the vapor condenses. The volume of the flask is the volume of the vapor, the boiling point of the water is the temperature of the vapor, and the atmospheric pressure is the pressure of the vapor. After removal from the water bath, the mass of the condensed vapor is determined. In one experiment, a student placed a flask with a volume of 295 mL into a boiling water bath (98°C) at a pressure of 752 torr. After removal from the bath, the flask contained 0.585 g of liquid. What was the molar mass of the liquid? 5.26 A student used the Dumas method (see Problem 5.25) to determine the molecular weight of an unknown liquid he was assigned to identify. At a temperature of 98°C and a pressure of 743 torr, 1.220 g of the liquid’s vapor occupied 375 mL. Determine the molecular weight of the unknown. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 39 5.27 An environmental scientist finds an unknown liquid. The first step she chose in order to identify the liquid was to determine its molecular weight using the Dumas method (see Problem 5.25). She found that at a temperature of 97°C and a pressure of 743 torr, 1.106 g of vapor occupied 295 mL. What did she find the molecular weight of the unknown to be? 5.28 A sample of neon gas (Ne) was placed in a 1250.0-mL container at a temperature of 17.00°C and a pressure of 795.0 mmHg. How many grams of neon were in the sample? 5.29 How many grams of carbon dioxide gas are in a container holding 3.50 L of carbon dioxide gas at 25°C and 895 torr? 5.30 The first binary compound of krypton to be prepared was KrF 2. A sample of this compound was prepared having a volume of 500.0 mL at a pressure of 795.0 mmHg and a temperature of 17.00°C. How many grams of KrF2 were in the sample? 5.31 (a) Hydrogen and chlorine react to produce gaseous hydrogen chloride (HCl) and heat. In one particular experiment, a total of 1250.0 mL of hydrogen chloride gas was produced at a temperature of 85.00°C and a pressure of 825.0 torr. How many grams of hydrogen chloride formed? (b) What is the percent yield if only 1.4530 g of hydrogen chloride formed? 5.5 Corresponds to BLBMWS Section 10.5 5.32 A sample of a gas occupying a volume of 5.72 L exerts a pressure of 725 mmHg. (a) What would be the volume in milliliters of the sample if you increased the pressure to 1.25 atm? (b) What would be the pressure in torr exerted by the gas if you decreased the volume to 3.76 L? 5.33 A sample of a gas occupies 10.55 L at a temperature of 25.85°C. (a) What will be the volume in liters of the gas if the temperature increased to 51.40°C? (b) What Celsius temperature is necessary to adjust the volume of the gas to 10.00 L? 5.34 A sample of a gas at a temperature of 15.5°C and a pressure of 0.895 atm occupies 14.3 L. (a) Determine the volume in milliliters of the gas at STP. (b) Determine the volume in liters of the gas at a pressure of 0.752 atm and a temperature of 31.0°C. (a) 1.21 x 104 mL (b) 17.9 L 5.35 A research student collected a sample of xenon gas in a 5.00-L container at a pressure of 225 mmHg and a temperature of 27°C. Later she found that the pressure had changed to 1.000 atm, and the volume had changed to 750.0 mL. What was the new Celsius temperature of the gas? 5.36 An engineer collected a sample of nitrogen gas in a 7.50 L container at a pressure of 575 torr and a temperature of 37°C. Later she found that the pressure had changed to 0.6000 atm, and the volume had changed to 7750.0 mL. What was the new Celsius temperature of the gas? 5.37 The initial pressure on a sample of oxygen gas was 795 torr. At this pressure, the sample occupied 1250.0 mL at 0°C. What was the final kelvin temperature of a sample of oxygen gas if the final volume was 1.000 L and the final pressure was 1.000 atm? 5.38 A sample of air had all of the oxygen removed to leave nearly pure nitrogen gas. Initially, the nitrogen gas had a volume of 15.00 L at 0.952 atm and 25°C. Later, the volume was 17.25 L and the pressure was 785 mmHg. What was the later temperature in degrees Celsius? 99°C 5.39 A chemist collected a 750.0-mL sample of air at a pressure of 745 mmHg and a temperature of 38°C. The sample was later compressed to a volume of 500.0 mL using a temperature of 273 K. What was the new pressure of the gas in atmospheres? 5.40 A 250.00-mL sample of helium gas was collected at a temperature of 25°C and a pressure of 1.15 atm. At what Celsius temperature will the sample of gas have a volume of 225.0 mL and a pressure of 725 torr? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 40 5.41 A sample of a gas occupies 15.55 L at a temperature of 27.85°C. At what temperature, in degrees Celsius, would the volume of the gas be 10.00 L? 5.42 A gas sample has a total pressure of 2.0 × 102 kPa. The sample contains 0.200 mol of hydrogen chloride, 0.150 mol of hydrogen bromide, 0.115 mol of hydrogen iodide, and 0.333 mol of argon. What is the partial pressure, in kilopascals, of each of the gases in the sample? 5.43 A mixture of gases made of 3.525 g C5H12, 2.528 g C3H8, and 3.056 g C4H10 was contained in a flask. The total pressure of the gases was 1.385 atm. Determine the partial pressure of each gas, in atmospheres. 5.6 Corresponds to BLBMWS Section 10.5 5.44 (a) A chemistry student analyzed a sample of an unknown hydrocarbon gas and found it contained 82.66 percent carbon and 17.34 percent hydrogen. Determine the empirical formula of the hydrocarbon. (b) A 1.50-L container held 3.4 g of the unknown hydrocarbon at 37°C and 1.00 atm. What was the molecular formula of the hydrocarbon? 5.45 The catalytic decomposition of hydrogen peroxide generates small quantities of oxygen gas by the reaction 2 H2O2(aq) 2 H2O(l) + O2(g) The generated oxygen acts as a disinfectant; this is why we use hydrogen peroxide to cleanse minor injuries. Calculate the number of grams of hydrogen peroxide necessary to generate 15.25 L of oxygen gas at 24.35°C, if the partial pressure of the oxygen is 745.0 torr. 5.46 The final step in the Ostwald process for the industrial synthesis of nitric acid is 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) How many liters of nitrogen dioxide (NO2) at 65.0°C and 3.75 atm are required to produce 275 kg of nitric acid? 5.47 Chlorine gas can be generated in the laboratory by reacting hydrochloric acid with manganese(IV) oxide: 4 HCl(aq) + MnO2(s) MnCl2(aq) + 2 H2O(l) + Cl2(g) In one experiment, 25.00 mL of 12.0 M hydrochloric acid was reacted with excess manganese(IV) oxide. The generated chlorine was collected over water at 26°C. (a) If the barometric pressure is 745 mmHg, what is the maximum volume in milliliters of chlorine gas that may be collected? The vapor pressure of water at 26°C is 25.2 mmHg. (b) What is the percent yield if only 792 mL of Cl2 formed? 5.48 In to the following reaction, how many grams of Mg(NH 2)2 are required to generate 5.00 L of NH3 gas at 27°C and 675 torr? 3 Mg(NH2)2(s) Mg3N2(s) + 4 NH3(g) 5.49 In the reaction below, how many grams of Al are required to generate 1.50 L of NH 3 at 35°C and 695 mmHg? 3 KNO3(aq) + 8 Al(s) + 5 KOH(aq) + 18 H 2O(l) 8 KAl(OH)4(aq) + 3 NH3(g) 5.50 According to the reaction below, how many grams of NH 2Cl would we use if we collected 12.3 L of N2 over water at 30.0°C and a total pressure of 795 mmHg? 2 NH2Cl(s) + N2H4(aq) 2 NH4Cl(aq) + N2(g) 5.51 After generating PH3 gas by the reaction shown below, you have collect 1.75 L of PH 3 gas over water at 17°C. If the total pressure of the gases is 775 mmHg, how many grams of H 3PO3 should decompose? 4 H3PO3(aq) 3 H3PO4(aq) + PH3(g) 5.52 Chemists use the reaction below to generate PH3 gas. In one experiment, a chemist collected 2.75 L of PH3 gas over water at 20.0°C with a total pressure of 785 torr. How many grams of KH 2PO2 did the chemist produce at the same time? P4(s) + 3 KOH(aq) + 3 H2O(l) PH3(g) + 3 KH2PO2(aq) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 41 5.53 A 2.75-L sample of H2S was stored at a temperature of 25°C with a pressure of 875 mmHg. How many grams of S could this sample produce in the following reaction? 5 H2S(g) + 2 H3AsO4(aq) 2 S(s) + As2S3(s) + 8 H2O(l) 5.54 A student used the reaction below to generate H2 gas. After some Si2H6 was reacted with an excess of H2O, she collected a total of 7.75 L of H2 over water at 30.0°C. The total pressure of the gas sample was 685 torr. How many grams of Si2H6 did she use in this reaction? Si2H6(aq) + 4 H2O(l) 2 SiO2(s) + 7 H2(g) 5.55 A researcher used the reaction below to generate H2 gas. In one experiment, he collected a total of 1.85 L of gas over water at 15°C. The total pressure of the gas was 895 torr. How many grams of B 4H10 did he generate while this amount of H2 formed? B6H12(s) + 6 H2O(l) B4H10(s) + 2 H3BO3(l) + 4 H2(g) 5.56 The reaction below may be used to generate (CN)2 gas. The two products of the reaction are much more stable than the highly unstable Cu(CN)2. If 2.85 L of (CN)2 produced by this reaction was collected over water at 40.0°C with a total pressure of 475 torr, how many grams of Cu(CN) 2 were decomposed? 2 Cu(CN)2(s) 2 CuCN(s) + (CN)2(g) 5.57 The reaction below for generating N2 gas is used as the means of inflation in some automobile airbags. In a test of this reaction, an 8.25-L sample of gas was collected over water at 25°C and at a total pressure of 875 mmHg. How many grams of NaN3 were used? 2 NaN3(s) 2 Na(s) + 3 N2(g) 5.58 A student in a general chemistry class used the reaction below to generate KClO 3. How many grams of KClO3 could be prepared from 8.75 L of Cl2 gas, if the gas was originally at a pressure of 1275 mmHg and a temperature of 57°C? 3 Cl2(g) + 6 KOH(aq) KClO3(aq) + 5 KCl(aq) + 3 H2O(l) 5.59 A chemistry graduate student used the reaction below to generate SiF4 gas. What volume of SiF4 gas could be prepared at 27°C and 550.0 torr from 45.3 g of XeF 6? 2 XeF6(s) + SiO2(s) 2 XeOF4(l) + SiF4(g) 3.14 L 5.60 Students use the reaction below to generate O2 gas. In one experiment, 8.25 L of gas was collected over water at 35°C and a total pressure of 895 mmHg. How many grams of Ba(IO 3)2 were used in this experiment? 5 Ba(IO3)2(s) Ba5(IO6)2(s) + 4 I2(s) + 9 O2(g) 5.61 After using the reaction below to generate hydrogen gas, you collect the H 2 over water at a temperature of 15°C. You collect a total of 12.5 L of gas with a total pressure of 795mmHg. How many grams of Al did you use to produce this much gas? 2 Al(s) + 6 HCl(aq) 2 AlCl3(aq) + 3 H2(g) 9.79 g Al 5.62 An engineer used the reaction below to generate SiF4 gas. How many liters of SiF4 gas could she generate by reacting 75.00 g of HF with an excess of SiO2 if the pressure of the SiF4 is 745 torr at a temperature of 27°C? SiO2(s) + 4 HF(aq) 2 H2O(l) + SiF4(g) 5.63 A student uses the reaction below to generate ClO2 gas. How many milliliters of ClO2 did he form by reacting 125.0 g of H2C2O4 with an excess of HClO3? The gas was collected as pure ClO2 (all other possible gaseous impurities were removed). The ClO2 was collected at 37°C and a pressure of 675 mmHg. 2 HClO3(aq) + H2C2O4(aq) 2 ClO2(g) + 2 H2O(l) + 2 CO2(g) 5.64 Chemists produce chlorine gas (Cl2) by the electrolysis of a salt-water solution. A 0.7500-L sample of chlorine was prepared in this manner; it had a pressure of 0.9500 atm and a temperature of 37.0°C. Later the volume had changed to 500.0 mL and the pressure had changed to 695.0 mmHg. What was the temperature at the new volume and pressure? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 42 5.7 Corresponds to BLBMWS Sections 10.7-10.8 5.65 A teacher gives a student two 2.0-L balloons. One balloon contains helium (He) gas, and the other balloon contains carbon dioxide (CO2) gas. The balloons are identical in size, temperature, and pressure. (a) How do the number of molecules in the balloon compare? (b) How do the densities of the gases in the balloons compare? (c) How does the speed of the gas molecules in the balloons compare? (d) How does the average kinetic energy of the two gases in the balloons compare? (e) Which of the two gases will effuse faster? 5.66 In an effusion experiment that was set up to determine the molecular weight of an unknown gas, 39.71 mL of the gas effused through a porous barrier in 262.0 s. When the experiment was repeated using oxygen gas, 47.92 mL of oxygen effused through the same barrier in 148.0 s. What was the molecular weight of the unknown gas? 5.67 An engineer submitted a sample of an unknown gas for analysis. One of the steps in the analysis was to determine the molecular weight of the unknown gas. To make this determination, lab technicians allowed the gas to effuse through a porous barrier and found that 54.87 mL of the gas effused in 372.0 s. A sample of chlorine gas under the same pressure and at the same temperature was then tested for comparison, and 75.45 mL of it effused in 275.0 s. What was the molecular weight of the unknown gas? 5.68 In an effusion experiment to determine the molecular weight of an unknown gas, 86.85 mL of the unknown gas effused through a porous barrier in 604.0 s. When the experiment was repeated using bromine vapor, 118.45 mL of bromine effused through the same barrier in 555.0 s. What was the molecular weight of the unknown gas? 352.1 g/mol 5.8 Corresponds to BLBMWS Section 10.9 5.70 Explain which of these gases is most likely to behave as an ideal gas under high pressure given the van der Waals constants shown below: Gas a (L2 atm/mol2) b (L/mol) CO2 3.658 0.04286 SO2 6.865 0.05679 O2 1.382 0.03186 5.71 You place a sample of 2.50 mol of chlorine gas (Cl2) in a 1.00-L steel container at 25°C. (a) Calculate the pressure exerted by the gas if it behaved ideally. (b) Calculate the pressure exerted by the gas if it did not behave ideally. The van der Waals constants for chlorine gas are a = 6.49 L2 atm/mol2 b = 0.0562 L/mol. (a) 61.1 atm (b) 30.6 atm 5.9 Summary 5.77 If the air in a particular urban area contains 175 g of suspended particulates per cubic meter, how many particles do people inhale in one breath? Assume the particles are spherical with a radius of 0.50 m and a density of 1.05 g/cm3. The average human breath is 0.50 L. 5.78 The safety standard for sulfur dioxide is 5 ppm. How much sulfur must burn to achieve this concentration in a room 5.11 Summary 5.83 A student collected a sample of oxygen gas in the laboratory by inverting a bottle of water in a tank of water at 25°C and bubbling oxygen gas, from the decomposition of potassium chlorate (KClO 3), into the inverted bottle until all the water was displaced. What was the partial pressure of the water vapor in the bottle? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 43 5.84 In the following reaction, how many grams of NaBH4 are required to produce 4.85 L of B 2H6 at a pressure of 685 torr and a temperature of 37°C? 3 NaBH4(s) + BF3(l) 3 NaF(s) + 2 B2H6(g) 5.85 Students use the reaction below to generate BF3 gas. How many grams of CaSO4 form when 3.35 L of BF3 is produced at 575 torr and 27°C? B2O3(s) + 3 H2SO4(aq) + 3 CaF2(s) 3 CaSO4(s) + 3 H2O(l) + 2 BF3(g) 5.86 A student produced hydrogen gas from the reaction of hydrochloric acid (HCl) with zinc metal (Zn), collecting the gas by the displacement of water from an inverted 500.0-mL flask. The temperature of the flask was 35.0 °C. The total pressure in the flask was 0.885 atm. Determine the partial pressure of hydrogen in the flask. 5.87 Hydrogen gas will react with molten sodium metal to produce sodium hydride (NaH). How many liters of hydrogen gas at 755 torr and 175°C are necessary to prepare 25.0 g of sodium hydride? 5.88 An evacuated 5.0-L flask is filled with 10.0 g of each of the following gases: hydrogen chloride (HCl), hydrogen bromide (HBr), and hydrogen sulfide (H 2S). The temperature of the flask is adjusted to 25.0°C. (a) Determine the partial pressure of each gas. (b) Determine the total pressure in the flask. 5.89 The final step in the Ostwald process for the industrial synthesis of nitric acid is 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) How many liters of nitrogen dioxide (NO2) at 75.35°C and 2.750 atm are required to produce 225.0 kg of nitric acid? 5.570 x 104 L 5.90 A sample of a partially combusted gas mixture was analyzed and found to contain 0.22 mol of methane (CH 4), 0.42 mol of carbon monoxide (CO), and 0.88 mol of carbon dioxide. The sample had a total pressure of 0.795 atm. Determine the partial pressure of each gas in the sample. 5.91 (a) Determine the mass in kilograms of ammonia gas in a 1.00 × 10 3-liter tank at 35°C and a pressure of 925 mmHg. (b) What would be the volume of this gas, in liters, at 27°C and 1.00 atm? 5.94 A student collected a sample of xenon gas in a 5.000-L container at a pressure of 225.0 mmHg and a temperature of 27.0°C. Later, she found that the pressure had changed to 1.000 atm, and the temperature had changed to 295 K. What was the new volume of the gas in milliliters? 5.95 The initial pressure on a sample of oxygen gas was 795.0 torr. At this pressure, the sample occupied 1.2500 L at 0.00°C. What was the final temperature in degrees Celsius of a sample of oxygen gas if the final volume was 1.000 L and the final pressure was 1.000 atm? –64.2 °C 5.96 A sample of carbon dioxide was prepared by burning pure carbon in oxygen gas. After the reaction, you found that 1.25 L of gas had been collected at a pressure of 1.000 atm, and that the burning had heated the sample to 127°C. Later, you found the volume to be 125 mL and the temperature 175°C. What was the pressure in millimeters of mercury at the new volume and temperature? 5.97 Hydrogen will reduce many metal oxides to the metal. A sample of a tungsten oxide with the general formula WmOn is reduced by this method. In one experiment, excess hydrogen reacted to produce 0.255 g of tungsten (W) metal and 159 mL of water vapor at 125°C and 0.855 atm. What is the formula of the tungsten oxide? 5.98 Hydrogen will reduce many metal oxides to the metal. A sample of an iron oxide undergoes reduction by this method. In one experiment, excess hydrogen reacted to produce 0.222 g of iron metal and 264 mL of water vapor at 135°C and 0.755 atm. What is the formula of the iron oxide? 5.99 Students use the reaction below to generate O2 gas. The O2 was collected at a temperature of 30.0 °C over water. The total pressure was 875 mmHg. If the students found that 1.75 L of O2 gas was generated, how many grams of KMnO4 were used? 2 KMnO4(s) K2MnO4(s) + MnO2(s) + O2(g) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 44 5.100 A student used the reaction below to generate O2 gas. A 5.25-L sample of O2 was prepared. The O2 was collected over water at a temperature of 25°C and under a total pressure of 745 torr. How many grams of KO 3 were required for the reaction? 4 KO3(s) + 2 H2O(l) 4 KOH(aq) + 5 O2(g) 5.101 In contact with air, sodium hydroxide (NaOH) slowly loses its purity by reacting with carbon dioxide (CO 2) to produce sodium carbonate (Na2CO3): 2 NaOH(s) + CO2(g) Na2CO3(s) + H2O(l) How many milliliters of carbon dioxide at 725 torr and 25.0°C will react with 2.50 g of sodium hydroxide? 5.102 As part of the process of identifying any unknown liquid, chemists must determine its molecular weight. A chemist who chose the Dumas method (see Problem 5.27) for this measurement found that at a temperature of 99°C and a pressure of 755 torr, 2.040 g of vapor occupied 384 mL. Determine the molecular weight of the unknown. 5.103 Oxalic acid (H2C2O4) decomposes when heated to carbon dioxide (CO2), carbon monoxide (CO), and water vapor. A graduate student heated a sample of oxalic acid until it completely decomposed. She collected the generated gases over water at 20.0 °C. The gas mixture occupied 2.75 L at 775 torr. The vapor pressure of water at 20.0°C is 17.5 torr. (a) Write a balanced equation for the decomposition of oxalic acid. (b) How many grams of oxalic acid were in the sample? 5.105 A chemist performed an analysis on a gas sample with a density of 1.80 g/L at 25°C and 0.955 atm and found it to be 52.2 percent carbon, 13.0 percent hydrogen, and 34.8 percent oxygen. Assuming there was only one compound in the gas sample, what is its molecular formula? 5.106 A chemist analyzes a gas sample with a density of 5.42 g/L at 35°C and 684 torr and found it to contain 63.15 percent carbon, 5.30 percent hydrogen, and 31.55 percent oxygen. What is the molecular formula of the gas? 5.107 An engineer submitted a sample of an unknown gas for analysis. The first step toward determining the molecular weight of the unknown gas was to allow the gas to effuse through a porous barrier. The technicians found that 30.90 mL of the gas effused in 408.0 s. A sample of nitrogen gas was then tested under the same pressure and at the same temperature, as a standard of comparison, and 45.84 mL of it effused in 152.0s. What was the molecular weight of the unknown gas? 5.108 The German zeppelin Hindenburg was destroyed over Lakehurst, New Jersey, on May 26, 1937. The zeppelin was over 800 ft long and had a diameter of about 135 ft. The hydrogen in the Hindenburg burst into flame, and over 30 people died in the disaster. The dirigible contained 7.2 × 10 6 ft3 of hydrogen gas at 0.985 atm and a temperature of 20.0°C. Calculate the mass of hydrogen in the Hindenburg. 5.109 Most aerosol cans contain a gas or gases under pressure. If you heat the can sufficiently, it will explode. If the pressure in such a can is 1.9 atm at 25°C, how high will the temperature need to be to cause the can to explode, assuming it will explode when the internal pressure reaches 3.1 atm? 5.110 Assume that gasoline is pure octane (C8H18) and that when it burns in an automobile engine, only carbon dioxide gas and water vapor form. (a) Write a balanced chemical equation for the complete combustion of octane in an automobile engine. (b) If a cylinder in an automobile engine has a volume of 525 cm 3, how many moles of oxygen would be present at 90.0°C and 0.995 atm? (c) Air is 20.95 percent oxygen. Calculate the maximum number of grams of octane that the oxygen in part b could completely burn. 5.111 The radioactive decay of uranium is a natural source of radon. This inert gas poses a radiation hazard when it diffuses out of the ground and accumulates in homes. In many parts of the United States, the radon in homes reaches 1.4 × 10–18 atm at 298 K. How many radon atoms are in a room measuring 12.5 ft × 10.0 ft × 8.0 ft? 5.112 Gaseous diborane (B2H6) spontaneously ignites in oxygen gas to form solid diboron trioxide and water vapor. (a) Write a balanced equation for this reaction. (b) In one experiment, a 10.00 g sample of diborane is mixed with 50.00 g of oxygen gas in a 1.00-L container. After the reaction has gone to completion, the temperature in the container is 127°C. What is the final partial pressure of each of the substances involved in the reaction? (c) What is the final mole fraction of each of the substances involved in the reaction? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 45 5.113 A chemist wishes to identify an unknown liquid and must therefore determine its molecular weight. She chose the Dumas method (see Problem 5.27) and found that at a temperature of 98°C and a pressure of 715 torr, 1.668 g of vapor occupied 415 mL. Determine the molecular weight of the unknown. 5.114 Name each of the following compounds: (a) Na2SO4 (b) KNO3 (c) CaCO3 (d) Mg3(PO4)2 (e) Al2O3 (f) Li2SO3 (g) NH4Br (h) BaCl2 (i) Sr(OH)2 (j) CsI 5.115 What is the formula of each of the following compounds: (a) sodium carbonate (b) calcium nitrate (c) potassium phosphate (d) magnesium sulfite (e) barium sulfate (f) aluminum hydroxide (g) lithium bromide (h) strontium hydrogen sulfate (i) rubidium acetate (j) ammonium nitrite 5.116 What is the name or the formula of each of the following compounds: (a) nitric acid (b) HNO 2 (c) H2SO4 (d) carbonic acid (e) HC2H3O2 (f) phosphoric acid (g) chromic acid (h) sulfurous acid (i) H 3AsO4 (j) H2C2O4 5.117 Assign charges to each of the following substances. Then combine the ions to form a stable ionic compound. Finally, give the name for each compound formed. (a) K, F (b) Ca, Se (c) Al, O (d) Ra, HSO 3 (e) Li, Cr2O7 (f) Na, HCO3 (g) Mg, CrO4 (h) Al, C2H3O2 (i) Sr, NO3 (j) NH4, PO4 5.118 Many chemicals are known by their common names. Give the chemical names for each of the following substances. (a) lime (CaO) (b) blue vitriol (CuSO4·5H2O) (c) saltpeter (KNO3) (d) baking soda (NaHCO3) (e) muriatic acid (HCl) (f) milk of magnesia (Mg(OH)2) (g) battery acid (H2SO4) (h) Epsom salts (MgSO4·7H2O) (i) smelling salts ((NH4)2CO3) (j) table salt (NaCl) 5.119 There are many proposed alternatives to hydrocarbon fuels. These alternative fuels will still produce nitrogen oxides when combusted. Why do these alternative fuels produce nitrogen oxides? 5.120 Write balanced chemical equations for each of the following reactions. (a) Sulfur, in coal, burns to produce sulfur dioxide gas. (b) Sulfur dioxide gas, from burning coal, reacts with atmospheric oxygen gas to form gaseous sulfur trioxide. (c) Gaseous sulfur trioxide, from the air oxidation of sulfur dioxide, dissolves in raindrops to form an aqueous solution of sulfuric acid. (d) Acid rain, containing an aqueous solution of sulfuric acid, falls on a marble (calcium carbonate) statue and forms solid calcium sulfate, gaseous carbon dioxide, and liquid water. 5.121 A particular automobile engine emits 145 L of exhaust at 85°C and 765 mmHg. If the exhaust contains 210 ppm of nitrogen oxide (NO), how many moles of nitrogen oxide are present? 5.122 When a glass of pure water was set on a table, the water was neither acidic nor basic. Later the water was tested and found to be acidic. Why was the water acidic? Chapter 6 6.1 Corresponds to BLBMWS Section 5.1 6.1 The joule is a common unit for the expression of energy. Express the joule in terms of SI base units. 6.2 List three common energy units. 6.3 You are conducting an experiment to measure the temperature change of ice melting in a beaker. You are in a laboratory, and you have a beaker containing ice, water, and a thermometer on the lab bench. What items constitute the system, and what items constitute the surroundings? What observations establish the state of the system? 6.2 Corresponds to BLBMWS Section 5.1 6.4 Show how using the equation for kinetic energy and the SI base units gives energy units as a result. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 46 6.5 (a) A baseball is heavier than a tennis ball. (a) If both balls are traveling with the same velocity, which ball has the greater kinetic energy? (b) How fast must a 0.0750-kg ball travel to have the same kinetic energy as a 0.175-kg ball traveling at 45.0 m/s? 6.6 You drop two books off a desk. Both books hit the floor at the same velocity. One book is twice as heavy as the other is. (a) Which book strikes the floor with more kinetic energy? (b) If the heavier book has twice the mass of the lighter book, how much faster must the lighter book be traveling for both books to have the same kinetic energy? 6.7 A 200.0-grain bullet may leave the barrel of a .44 Magnum pistol with a velocity as high as 1475 ft/s. (7000 grains is exactly equal to 1 pound.) (a) Calculate the maximum velocity in m/s. (b) Calculate the kinetic energy of the bullet in joules. 6.8 A 32.0-grain rifle bullet may leave the barrel of a .204 Ruger with a velocity of 4225 ft/s. (7000 grains is exactly equal to 1 pound.) (a) Calculate the maximum velocity in m/s. (b) Calculate the kinetic energy of the bullet in Joules. 6.9 What is the sign of the indicated variable in each of the following situations? (a) The internal energy increases. (b) The system loses heat. (c) The system does work on the surroundings. 6.10 What is the sign of the indicated variable in each of the following situations? (a) The system loses internal energy. (b) The system absorbs heat from the surroundings. (c) The surroundings do work on the system. 6.11 Determine how much the internal energy changes in each of the following cases: (a) A balloon bursts, and the released air expands without any significant heat exchange and does 125 J of work on the surroundings. (b) A pan of water on a stove absorbs 1250 J of heat. (c) A sample of a gas is compressed. During compression, the sample has 375 Joules of work done on it, and 125 Joules of heat energy are removed from the sample. 6.3 Corresponds to BLBMWS Section 5.2 6.12 State the first law of thermodynamics. 6.13 In your own words, define a state function. Give two examples of state functions. 6.14 If the pressure is in pascals and the volume is in liters, what conversions are necessary to determine pressurevolume work in joules? 6.15 How does the volume change when a system does pressure-volume work on the surroundings? 6.16 Decide whether each of the following processes is exothermic or endothermic. (a) Water in a water heater becomes warmer. (b) Water in a freezer turns into ice. (c) Water in a glass on a table evaporates. (d) A sample of charcoal burns. (e) When ice is removed from the freezer; it melts. 6.17 A balloon expands against a constant pressure of 1.00 atm. The volume of the balloon increases in volume from 0.500 L to 0.750 L. Determine the amount of pressure-volume work in Joules. 6.4 Corresponds to BLBMWS Sections 5.3 and 5.5 6.18 List the common units for each of the following properties. (a) heat capacity, (b) specific heat capacity, (c) molar heat capacity. 6.19 What additional information is necessary in each of the following cases? (a) You wish to determine the joules from the heat capacity. (b) You wish to determine the joules from the specific heat capacity. (c) You wish to determine the joules from the molar heat capacity. 6.20 Which type of calorimeter directly yields the enthalpy change? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 47 6.21 A piece of lead metal weighing 60.80 g was heated. The lead absorbed 235 J, and the temperature increased from 20.00 °C to 50.20 °C. Calculate the specific heat of lead. 6.22 Determine the specific heat of an unknown metal from the following data. A 39.2-g sample (at 61.67 °C) was dropped into 53.4 g of water (at 20.00 °C). The final temperature was 30.00 °C. 6.23 The specific heat of water is 4.184 J/g• °C. or 4.184 J/g•K. (a) Calculate the heat capacity of 575 g of water. (b) Calculate the enthalpy change when you heat 2.50 kg of water by 25.0 °C. (c) Calculate the enthalpy change when 1.750 kilograms of water cool from 51.35 °C to 26.25 °C. 6.24 Gold has a specific heat of 0.129 J/g• °C. (a) What is the specific heat of gold in J/g•K? (b) Calculate the change in enthalpy when 0.3251 troy ounces of gold cools from 37.0 °C to 25.0 °C. (1 troy ounce = 31.103 g) 6.25 A coffee-cup calorimeter, as illustrated in the textbook, is an inexpensive alternative to a bomb calorimeter, also illustrated in the textbook. Water is placed in a coffee-cup calorimeter, and some solid sodium hydroxide (NaOH) is added. The process of dissolving the sodium hydroxide results in an enthalpy change. Determine the enthalpy change, in kJ/mole NaOH, if the addition of 8.91 g of sodium hydroxide to 100.0 g of water causes the temperature to change from 22.4 °C to 44.6 °C. Assume that the specific heat of the solution is the same as that of pure water. –45.4 kJ/mol 6.26 When a 12.4-g sample of sodium nitrate dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature changes from 25.30 °C °C to 22.51 °C °C. Determine the enthalpy change in kJ/mol of sodium nitrate for the dissolution of sodium nitrate in water. Assume that the specific heat of the solution is the same as that of pure water. 6.27 An ethanol (C2H5OH) sample weighing 2.49 g was placed in a bomb calorimeter, and burned according to the following equation: C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(l) The total heat capacity of the calorimeter was 8.43 kJ/ °C. The reaction raised the temperature from 22.50 °C to 30.14 °C. (a) Calculate the heat of combustion for ethanol in kilojoules per gram of ethanol. (b) Calculate the heat of combustion in kilojoules per mole of ethanol. 6.28 A sample of naphthalene (C10H8) weighing 1.542 g was burned in a bomb calorimeter. The reaction caused the temperature of the bomb calorimeter to increase from 21.37 °C to 27.46 °C. The heat of combustion of naphthalene is 40.14 kJ/g. (a) Calculate the total heat capacity of the calorimeter. (b) If there were a total of 2.000 kg of water in the calorimeter, what would be the heat capacity of the empty calorimeter? (c) In a second experiment, only 1.750 kg of water was placed in the calorimeter, and a 1.385-g sample of naphthalene was burned. Calculate the temperature change in the second experiment. 6.29 Naphthalene is a component of many mothballs. To measure its heat of combustion, a 2.870-g sample was combined with an excess of O2 and burned in a bomb calorimeter. After the reaction, the temperature of the calorimeter had increased from 23.00 °C to 34.34 °C. The calorimeter contained 2.000 kg of water. The heat capacity of the calorimeter was 1.80 kJ/ °C. Determine the heat of reaction in kJ/mol naphthalene for the reaction C10H8(s) + 12 O2(g) 10 CO2(g) + 4 H2O(l) 6.30 The compound B5H9 was investigated at one time as a potential rocket fuel, because of the energy generated by the following reaction: 2 B5H9(g) + 12 O2(g) 5 B2O3(s) + 9 H2O(g) In order to determine the heat of reaction, 0.400 g of B 5H9 was burned with an excess of O2 in a bomb calorimeter. The heat capacity of the calorimeter was 1.840 kJ/ °C. The calorimeter contained 1704 g of water, and the temperature increased from 25.00 °C to 28.14 °C. Calculate the heat of reaction per mole of B5H9. –4.44 × 103 kJ/mol © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 48 6.31 The reaction of 3.986 g of Fe2O3 with an excess of Al was carried out in a bomb calorimeter. The heat capacity of the calorimeter was 1.96 kJ/ °C, and the calorimeter contained 1.97 kg of water. After the reaction, it was determined that the temperature had increased from 25.00 to 27.58 °C. Determine the heat of reaction per mole of Al for the following reaction. Fe2O3(s) + 2 Al(s) Al2O3(s) + 2 Fe(l) 6.32 The enthalpy of combustion for benzoic acid (HC7H5O2) is –3221.6 kJ/mol. A 1.8476-g sample of benzoic acid was burned in a bomb calorimeter. The temperature of the calorimeter rose by 3.56 °C. If the calorimeter contained exactly 2.000 kg of water, what was the heat capacity of the empty calorimeter? 6.33 Lactic acid (C3H6O3), generated in the body by the partial oxidation of glucose, can be further oxidized in the body to produce energy. Combustion of a 5.24-g sample with an excess of O2 in a bomb calorimeter raised the temperature of the calorimeter from 22.000 °C to 25.600 °C. The calorimeter contained 2.000 kg of water. The heat capacity of the calorimeter was 13.33 kJ/ °C. Determine the heat of reaction in kJ/mole lactic acid for the following reaction C3H6O3(s) + 3 O2(g) 3 CO2(g) + 3 H2O(l) 6.34 Natural gas is mostly CH4. Combustion of a 0.3200-g sample with an excess of O2 in a bomb calorimeter increased the temperature of the calorimeter from 20.00 °C to 23.12 °C. The calorimeter contained 1.000 kg of water. The heat capacity of the calorimeter was 958 J/ °C. Determine the heat of reaction in kJ/mol CH 4 for the following reaction CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) 6.35 Butane (C4H10) is used as a fuel. Combustion of a 2.000-g sample with an excess of O2 increased the temperature of a bomb calorimeter from 23.00 °C to 34.34 °C. The calorimeter contained 1.000 kg of water. The heat capacity of the calorimeter was 3.88 kJ/ °C. Determine the heat of reaction in kJ/mol C 4H10 for the following reaction 2 C4H10(g) + 13 O2(g) 8 CO2(g) + 10 H2O(l) 6.36 A sample of C6H6(l) weighing 3.500 g was burned with an excess of oxygen in a bomb calorimeter, raising the temperature of the calorimeter from 25.00 °C to 36.33 °C. The calorimeter contained 2.000 kg of water. The heat capacity of the calorimeter was 3.68 kJ/ °C. From these data, determine the heat of reaction for 2 C6H6(l) + 15 O2(g) 12 CO2(g) + 6 H2O(l) 6.5 Corresponds to BLBMWS Sections 5.4-5.6 6.37 Why is it acceptable to use fractional coefficients in thermochemical equations but not in normal chemical equations? 6.38 State Hess's law in your own words. 6.39 How can a person driving from New York City to San Francisco illustrate Hess's law? 6.40 What is the expected sign for H in each of the following cases? (a) H2O(l) H2O(g) (b) CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) Methane (CH4) is natural gas. (c) CO2(g) + 2 H2O(l) CH4(g) + 2 O2(g) (d) NH4NO3(s) NH4NO3(aq) The temperature of the solution is lowered. (e) CO2(g) CO2(s) 6.41 The following reaction releases 198 kJ: 2 SO2(g) + O2(g) 2 SO3(g) What is the enthalpy change when 35.00 g of SO2(g) react? –54.1 kJ © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 49 6.42 Ammonia (NH3) gas burns with oxygen gas (O2) in the presence of a catalyst to form water vapor and nitrogen (N2) gas. A thermochemical equation for this reaction is 4 NH3(g) + 3 O2(g) 6 H2O(g) + 2 N2(g) H = –1267 kJ (a) Determine the enthalpy change for 2 NH3(g) + 3/2 O2(g) 3 H2O(g) + N2(g) H = ? (b) Determine the enthalpy change for 8 NH3(g) + 6 O2(g) 12 H2O(g) + 4 N2(g) H = ? (c) Determine the enthalpy change for 6 H2O(g) + 2 N2(g) 4 NH3(g) + 3 O2(g) H = ? 6.43 The combustion of octane in gasoline proceeds by the following reaction: 2 C8H18(l) + 25 O2(g) 16 CO2(g) + 18 H2O(l) H = –10942 kJ How much energy is released when 175 g of CO2(g) are formed by this reaction? –2.72 × 103 kJ 6.44 Hydrogen gas burns in oxygen gas according to the reaction 2 H2(g) + O2(g) 2 H2O(l) H = –571.7 kJ (a) Is this an endothermic or an exothermic process? (b) What is the energy change when 0.5000 mol of hydrogen are combusted in the above reaction? Assume the pressure remains constant. (c) What is the energy change when 2.75 g of hydrogen burns at constant pressure? (d) Calculate the number of grams of water produced by an energy change of 175 kJ. (e) Calculate the energy change when 15.0 g of water decomposes to the elements at constant pressure. 6.45 Carbon monoxide reacts with nitrogen oxide as follows 2 CO(g) + 2 NO(g) 2 CO2(g) + N2(g) H = –746.6 kJ (a) Calculate the enthalpy change when 0.250 mol of carbon dioxide form. (b) Calculate the enthalpy change when 50.0 g of NO(g) are consumed. (c) What would be the enthalpy change if 0.375 mol of CO(g) are produced by the reverse of the above reaction? 6.46 The following thermochemical equation is endothermic by 90.3 kJ: Calculate the enthalpy change when 25.00 g of NO(g) decompose. N2(g) + O2(g) 2 NO(g) 6.47 The following thermochemical equation is exothermic by 1037 kJ: 2 H2(g) + 3 O2(g) 2 SO2(g) + 2 H2O(g) Calculate the enthalpy change when 125.0 g of SO2(g) gas react as follows: 2 SO2(g) + 2 H2O(g) 2 H2(g) + 3 O2(g) 6.48 Under standard conditions, 0.2000 mol of OF2(g) produced 64.8 kJ in the reaction OF2(g) + H2O(g) O2(g) + 2 HF(g) Calculate the enthalpy change for this reaction in terms of kJ/mol OF2. 6.49 The following exothermic reaction is used in the altitude-control engines of the space shuttle: 4 CH6N2(l) + 5 N2O4(l) 4 CO2(g) + 3 H2O(l) + 5 N2(g) The molar heat of reaction is 1384 kJ. What is the energy change when 25 g of N 2O4(l) reacts? 6.50 The compound HC2H3O2 combusts as follows: HC2H3O2(l) + 3 O2(g) 2 CO2(g) + 2 H2O(l) The reaction is exothermic and produces 871.5 kJ/mol HC2H3O2. How many grams of O2 are required for this reaction if the enthalpy change is to be –1000.0 kJ? 6.51 How many grams of C2H4(g) must be burned to produce 3250.0 kJ of heat? The heat of combustion of C 2H4(g) is –1410.0 kJ/mol C2H4. 64.663 g © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 50 6.52 Use Hess's law to determine H for the reaction N2(g) + 2 H2(g) N2H4(l) Use the following equations N2H4(l) + O2(g) N2(g) + 2 H2O(l) H = –622.2 kJ 2 H2(g) + O2(g) 2 H2O(l) H = –571.6 kJ H = +50.6 kJ 6.53 Using the thermochemical equations 2 H2(g) + O2(g) 2 H2O(l) N2O5(g) + H2O(l) 2 HNO3(l) N2(g) + 3 O2(g) + H2(g) 2 HNO3(l) Determine H for the following reaction: 2 N2(g) + 5 O2(g) 2 N2O5(g) H = –571.6 kJ H = –76.6 kJ H = –348.2 kJ 6.54 Using the thermochemical equations 2 Al(s) + 6 HCl(aq) 2 AlCl3(aq) + 3 H2(g) H = –1049 kJ HCl(g) HCl(aq) H= –75.2 kJ H2(g) + Cl2(g) 2 HCl(g) H = –184.6 kJ AlCl3(s) AlCl3(aq) H = –323 kJ Determine H for the formation reaction for solid aluminum chloride: 2 Al(s) + 3 Cl2(g) 2 AlCl3(s) H = –1408 kJ 6.55 Using the thermochemical equations 2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(l) H = –2600. kJ 2 C2H6(g) + 7 O2(g) 4 CO2(g) + 6 H2O(l) H = –3120. kJ H2(g) + 1/2 O2(g) H2O(l) H = –285.8 kJ Calculate the heat of reaction for: C2H2(g) + 2 H2(g) C2H6(g) 6.56 Calculate the heat of reaction for W(s) + C(s) WC(s) Using the thermochemical equations: 2 W(s) + 3 O2(g) 2 WO3(s) H = –1680.6 kJ 2 WC(s) + 5 O2(g) 2 WO3(s) + 2 CO2(g) H = –2391.6 kJ C(s) + O2(g) CO2(g) H = –393.51 kJ 6.57 Calculate the heat of reaction for 2 N2(g) + 5 O2(g) 2 N2O5(g) Use the following thermochemical equations N2(g) + 3 O2(g) + 1 H2(g) 2 HNO3(aq) N2O5(g) + H2O(g) 2 HNO3(aq) 2 H2(g) + O2(g) 2 H2O(g) H = –413.14 kJ H = 218.4 kJ H = –483.64 kJ 6.58 Find H for the reaction: C(s) + 2 H2(g) CH4(g) Use the following thermochemical equations: C(s) + O2(g) CO2(g) H = –393.5 kJ H2(g) + 1/2 O2(g) H2O(l) H = –285.8 kJ CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) H = –890.28 kJ 6.6 Corresponds to BLBMWS Section 5.7 6.61 Define a standard heat of formation. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 51 6.62 For each of the following substances, derive a balanced thermochemical equation for the standard heat of formation of one mole of the substance from its constituent elements. Assume all materials are in their standard states. Use Appendix C to determine the enthalpy change for each reaction. (a) solid calcium iodide (CaI 2(s)) (b) gaseous sulfur trioxide (SO3(g)) (c) solid sodium carbonate (Na2CO3(s)) (d) liquid ethanol (C2H5OH(l)) (e) solid sodium bicarbonate (NaHCO3(s)). 6.63 For each of the following substances, derive a balanced thermochemical equation for the standard heat of formation of one mole of the substance from its constituent elements. Assume all materials are in their standard states. Use Appendix C to determine the enthalpy change for each reaction. (a) solid silver chloride (b) gaseous hydrogen bromide (c) solid aluminum oxide (d) liquid sulfuric acid (e) solid barium chloride dihydrate. (a) Ag(s) + 1/2 Cl2(g) AgCl(s) ΔH = –107.9 kJ (b) 1/2 H2(g) + 1/2 Br2(l) HBr(g) ΔH = –36.23 kJ (c) 2 Al(s) + 3/2 O2(g) Al2O3(s) ΔH = –1669.8 kJ (d) H2(g) + S(s) + 2 O2(g) H2SO4(l) ΔH = –813.989 kJ (e) Ba(s) + Cl2(g) + 2 H2(g) + O2(g) BaCl2•2H2O(s) ΔH = –1460 kJ 6.64 Calculate the standard heat of formation for HC 2H3O2(l) using the following thermochemical equations C(s) + O2(g) CO2(g) H = –393.5 kJ H2(g) + 1/2 O2(g) H2O(l) H = –285.8 kJ HC2H3O2(l) + 2 O2(g) 2 CO2(g) + 2 H2O(l) H = –871 kJ 6.65 Using the following thermochemical equations, calculate the standard heat of formation for C 2H2(g) 2 C2H2(g) + 5 O2(g) 4 CO2(g) + 2 H2O(l) H = –2547.6 kJ C(s) + O2(g) CO2(g) H = –393.5 kJ 2 H2(g) + O2(g) 2 H2O(l) H = –571.66 kJ 6.66 Calculate the heat of formation for ZnO, using the following thermochemical equations: Zn(s) + 2 HCl(aq) ZnCl2(aq) + H2(g) H = –152.4 kJ ZnO(s) + 2 HCl(aq) ZnCl2(aq) + H2O(l) H = –90.2 kJ 2 H2(g) + O2(g) 2 H2O(l) H = –571.6 kJ 6.67 Determine the enthalpy change for each of the following: (a) 2 ZnS(s) + 3 O2(g) 2 ZnO(s) + 2 SO2(g) (b) C6H12(l) + 9 O2(g) 6 CO2(g) + 6 H2O(l) (c) CaO(s) + H2O(l) Ca(OH)2(s) 6.68 Determine the enthalpy change for each of the following: (a) Ca(OH)2(s) CaO(s) + H2O(g) (b) 2 Cu(s) + O2(g) 2 CuO(s) (c) C6H12(l) + 6 O2(g) 6 CO(g) + 6 H2O(l) 6.69 Determine the enthalpy change for each of the following: (a) CuO(s) + Cu(s) Cu2O(s) (b) C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(l) (c) 4 NH3(g) + 3 O2(g) 2 N2(g) + 6 H2O(l) 6.70 Determine the enthalpy change for each of the following: (a) Ca(OH)2(s) + H2SO4(aq) CaSO4(s) + 2 H2O(l) (b) 2 Zn(s) + O2(g) 2 ZnO(s) (c) C2H5OH(l) + 2 O2(g) 2 CO(g) + 3 H2O(l) 6.71 Ethyl alcohol (C2H5OH(l)) undergoes the following combustion reaction: C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(l) ∆H = –1366.8 kJ What is the enthalpy change when 5.00 g of ethyl alcohol burns according to this reaction? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 52 6.72 Determine the enthalpy change for each of the following: (a) P4O10(s) + 6 H2O(l) 4 H3PO4(aq) (b) 2 C4H10(g) + 13 O2(g) 8 CO2(g) + 10 H2O(g) (c) Pb(NO3)2(s) + 2 KI(s) PbI2(s) + 2 KNO3(s) 6.73 At one time, manganese metal was produced by the reaction 4 Al(s) + 3 MnO2(s) 2 Al2O3(s) + 3 Mn(s) Determine H° for this reaction using data from Appendix C. 6.74 Determine the enthalpy change for the following reactions using information in Appendix C: (a) H2(g) + Br2(l) 2 HBr(g) (b) 2 NaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g) (c) BaO(s) + H2SO4(l) BaSO4(s) + H2O(l) (d) P4O10(s) + 6 H2O(l) 4 H3PO4(aq) (e) 8 CO2(g) + 10 H2O(l) 2 C4H10(g) + 13 O2(g) 6.75 Diborane (B2H6) reacts with oxygen as follows: B2H6(g) + 3 O2(g) B2O3(s) + 3 H2O(g) H° = –2035 kJ Using the above reaction and Appendix C, determine Hf° for diborane. 6.7 Corresponds to BLBMWS Section 11.4 6.76 Identify the type of phase transition occurring in each of the following changes: (a) dew forms on grass (b) ice cubes in a freezer slowly disappear (c) when warmed in a pan on a stove, butter changes to a liquid (d) water in a glass slowly disappears (e) gaseous carbon dioxide forms dry ice (solid carbon dioxide). 6.77 Two pans of water are placed on a stove, and the heat is turned on high. One pan contains 1 L of water, and the other contains 2 L of water. (a) If both pans are heated at the same rate, which will boil first? (b) If the water in the pan with less water is boiling at 100 °C, what is the temperature of boiling water in the other pan? (c) Once both pans begin to boil, the heat is reduced under one pan so that the water in it is barely boiling, while the other is left on high and boils vigorously. How do the temperatures of the water in the two pans compare? 6.78 In the desert, water may be cooled by evaporation. The evaporation of water from the surface of a water bag cools the liquid in the bag. Calculate the number of milliliters of water that may be cooled from 40.0 °C to 25 °C by the evaporation of 15 g of water. The density of water is 1.0 g/cm3, the specific heat of water is 4.18 J/g•K, and the heat of vaporization of water is 41 kJ/mol. 6.79 Cesium metal melts at 28 °C and boils at 669 °C. The enthalpy of fusion of cesium is 2.092 kJ/mol, and its enthalpy of vaporization is 68.28 kJ/mol. The specific heats of solid, liquid, and gaseous cesium are 0.233 J/g•K, 0.243 J/g•K, and 0.156 J/g•K, respectively. How much heat is required to convert 175.0 g of cesium at 0.0 °C to the vapor phase at 769 °C? 123.8 kJ 6.80 Gallium (Ga) melts at 29 °C and boils at 2403 °C. The enthalpy of fusion of gallium is 5.590 kJ/mol, and its enthalpy of vaporization is 295.8 kJ/mol. The specific heats of solid, liquid, and gaseous gallium are 0.381 J/g•K, 0.409 J/g•K, and 0.364 J/g•K, respectively. How much heat is required to convert 135.0 g of gallium at 0.00 °C to the vapor phase at 2600. °C? 6.81 Lead melts at 327 °C and boils at 1740. °C. The enthalpy of fusion of lead is 5.121 kJ/mol, and its enthalpy of vaporization is 177.8 kJ/mol. The specific heats of solid, liquid, and gaseous lead are 0.129 J/g•K, 0.154 J/g•K, and 0.100 J/g•K, respectively. How much heat is required to convert 1500.0 g of lead at 127 °C to the vapor phase at 1850. °C? 6.82 Xenon melts at –112 °C and boils at –107 °C. The enthalpy of fusion of xenon is 3.096 kJ/mol, and its enthalpy of vaporization is 12.64 kJ/mol. The specific heats of solid, liquid, and gaseous xenon are 0.159 J/g•K, 0.339 J/g•K, and 0.159 J/g•K, respectively. How much heat is required to convert 175.0 g of xenon at –162 °C to the vapor phase at 0.00 °C? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 53 6.83 Sulfur dioxide melts at –73 °C and boils at –10. °C. The enthalpy of fusion of sulfur dioxide is 8.619 kJ/mol, and its enthalpy of vaporization is 25.73 kJ/mol. The specific heats of liquid and gaseous sulfur dioxide are 0.995 J/g•K and 0.622 J/g•K, respectively. How much heat is required to convert 2.50 kg of solid sulfur dioxide at the melting point to the vapor phase at 60. °C? 6.84 Hydrogen iodide melts at –51 °C and boils at –35 °C. The enthalpy of fusion of hydrogen iodide is 2.871 kJ/mol, and its enthalpy of vaporization is 44.11 kJ/mol. The specific heats of liquid and gaseous hydrogen iodide are 0.365 J/g•K and 0.228 J/g•K, respectively. How much heat is required to convert 220.0 g of solid hydrogen iodide at the melting point to the vapor phase at 0.0 °C? 6.85 Benzene (C6H6) melts at –6 °C and boils at 80.0 °C. The enthalpy of fusion of benzene is 9.937 kJ/mol, and its enthalpy of vaporization is 42.90 kJ/mol. The specific heats of liquid and gaseous benzene are 1.74 J/g•K and 1.05 J/g•K, respectively. How much heat is required to convert 150.0 g of solid benzene at the melting point to the vapor phase at 125 °C? 6.86 Toluene (C7H8) melts at –95 °C and boils at 111 °C. The enthalpy of fusion of toluene is 6.61 kJ/mol, and its enthalpy of vaporization is 39.2 kJ/mol. The specific heats of liquid and gaseous toluene are 1.69 J/g•K and 1.13 J/g•K, respectively. How much heat is required to convert 235.0 g of solid toluene at the melting point to the vapor phase at 181 °C? 6.87 Dimethyl sulfide ((CH3)2S) melts at –98 °C and boils at 37 °C. The enthalpy of fusion of dimethyl sulfide is 7.97 kJ/mol, and its enthalpy of vaporization is 28.21 kJ/mol. The specific heats of liquid and gaseous dimethyl sulfide are 1.91 J/g•K and 1.16 J/g•K, respectively. How much heat is required to convert 375.0 g of solid dimethyl sulfide at the melting point to the vapor phase at 77 °C? 6.88 Ethyl ether ((C2H5)2O) melts at –116 °C and boils at 35 °C. The enthalpy of fusion of ethyl ether is 7.26 kJ/mol, and its enthalpy of vaporization is 29.06 kJ/mol. The specific heats of liquid and gaseous ethyl ether are 2.32 J/g•K and 1.46 J/g•K, respectively. How much heat is required to convert 425.0 g of solid ethyl ether at the melting point to the vapor phase at 85 °C? 6.89 Acetone (C3H6O) melts at –95 °C and boils at 56 °C. The enthalpy of fusion of acetone is 5.683 kJ/mol, and its enthalpy of vaporization is 31.97 kJ/mol. The specific heats of liquid and gaseous acetone are 2.18 J/g•K and 1.30 J/g•K, respectively. How much heat is required to convert 600.0 g of solid acetone at the melting point to the vapor phase at 66 °C? 6.8 Corresponds to BLBMWS Section 13.3 6.90 How does the solubility of a gas vary with temperature? 6.91 How does thermal pollution affect the ability of fish to survive in a river? 6.92 At 20 °C the Henry's law constants for nitrogen (N2) and oxygen (O2) are 6.9 × 10–4 M/atm and 1.38 × 10–3 M/atm, respectively. The air in a diving bell is under a pressure of 10.0 atm, at 20 °C. The mole fraction of nitrogen in air is 0.781, and the mole fraction of oxygen is 0.209 (neglecting water vapor). What is the solubility of each gas? 6.93 If the air pressure is 1.00 atm and the mole fraction of oxygen in the atmosphere is 0.209. Calculate the molar solubility of oxygen in the water in a lake at 20°. At this temperature, the Henry's law constant for oxygen is 1.38 × 10–3 M/atm. 6.9 Corresponds to BLBMWS Section 5.8 6.94 What is the relationship between (a) a nutritional calorie and a calorie, and (b) a nutritional calorie and a joule? 6.95 A package of a particular brand of instant oatmeal contains 2 g of fat, 34 g of carbohydrate, and 5 g of protein. Fats typically produce 38 kJ/g, and both proteins and carbohydrates typically produce 17 kJ/g. How many nutritional calories (Calories) are present in the package of instant oatmeal? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 54 6.96 A package of a particular brand of a microwavable Chinese food contains 10.0 g of fat, 87.0 g of carbohydrate, and 12.0 g of protein. Fats typically produce 38 kJ/g, and both proteins and carbohydrates typically produce 17 kJ/g. How many nutritional calories (Calories) are present in the package of Chinese food? 6.97 Nitrogen may be oxidized in automobile engines by the following reaction: N2(g) + O2(g) 2 NO(g) H = 180.6 kJ How many grams of nitrogen are required to absorb 125 kJ? 6.98 The reaction for the combustion of ethanol (C2H5OH) is C2H5OH(l) + 3 O2(g) 2 CO2(g) + 3 H2O(g) (a) Determine the heat of combustion. (b) A bottle of wine is 12.0 % alcohol by mass. The density of the wine is 1.090 g/mL. How many Calories does the alcohol in a 1.00 qt bottle of this wine contribute? 6.99 Octane is used in gasoline. In order to measure its heat of combustion, a 3.06-g sample was combined with an excess of O2 and ignited in a bomb calorimeter. The reaction raised the temperature of the calorimeter by 3.72 °C. The calorimeter contained 3.000 kg of water. The heat capacity of the calorimeter was 30.15 kJ/ °C. Determine the heat of reaction in kJ/mol octane for the reaction 2 C8H18(l) + 25 O2(g) 16 CO2(g) + 18 H2O(l) 6.100 Propane gas (C3H8) is sometimes used as a fuel. In order to measure its energy output as a fuel a 1.860-g sample was combined with an excess of O2 and ignited in a bomb calorimeter causing the temperature of the calorimeter to increase from 25.000 °C to 26.061 °C. The calorimeter contained 1.000 kg of water. The heat capacity of the calorimeter was 4.643 kJ/ °C. Determine the heat of reaction in kJ/mol propane. The reaction was C3H8(l) + 5 O2(g) 3 CO2(g) + 4 H2O(l) 6.101 Fructose is a sugar found in fruits. In order to measure its heat of combustion a 2.50-g sample was combined with an excess of O2 and ignited in a bomb calorimeter. The reaction raised the temperature of the calorimeter from 23.000 °C to 24.175 °C. The calorimeter contained 2.000 kg of water. The heat capacity of the calorimeter was 21.33 kJ/ °C. Determine the heat of reaction in kJ/mol fructose for the reaction C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) 6.102 Three types of oatmeal are described on the box of a variety pack of instant oatmeal. Determine the nutritional calories available from a serving of each type. Protein Fat Carbohydrate (a) Maple and Brown Sugar 4g 2g 33 g (b) Apple and Cinnamon 3g 1.5 g 27 g (c) Cinnamon and Spice 5g 2g 34 g 6.10 Summary 6.103 Unlike other chemical equations, thermochemical equations must contain the physical states of all substances involved. Why must this information be included? 6.104 Write a thermochemical equation for the standard heat of formation of solid sodium carbonate (Na 2CO3). 2 Na(s) + C(s) + 3/2 O2(g) Na2CO3(s) ∆Hf° = –1130.8 kJ 6.105 Write a thermochemical equation for the standard heat of formation of solid sodium hydrogen carbonate (NaHCO3). 6.106 A 100.0-g sample of water is heated to boiling, 100.00 °C, and removed from the heat source. A 45.0 g sample of manganese, at 25.00 °C, was immediately dropped into the hot water. The final temperature of the water plus manganese was 98.49 °C. Assuming no heat was lost, what is the specific heat of manganese? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 55 6.107 A 25.00-g sample of chromium metal was heated to 75.00 °C. This sample was clamped in contact with a 47.00-g sample of beryllium metal at 25.00 °C. The specific heat of chromium metal is 0.450 J/g•°C, and the specific heat of beryllium metal is 1.82 J/g•°C. Assuming no heat is lost to the surroundings, what was the final temperature of the two metals? 6.108 A 15.00 g sample of lead metal was heated to 65.00 °C. This sample was clamped in contact with a 27.00 g sample of magnesium metal at 25.00 °C. The specific heat of lead metal is 0.127 J/g °C, and the specific heat of magnesium metal is 1.024 J/g °C. Assuming no heat is lost to the surroundings, what was the final temperature of the two metals? 6.109 During respiration glucose (C6H12O6) is oxidized to carbon dioxide and water. The overall reaction may be simplified to C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(l) H = –2802.7 kJ (a) Is the enthalpy of the reactants higher or lower than that of the products? (b) Is this an endothermic or an exothermic reaction? (c) Calculate the enthalpy change when 2.500 g of glucose react. (d) Calculate the enthalpy change when 2.500 g of carbon dioxide gas are formed. (e) How many grams of glucose must be consumed to furnish 1000. kJ of energy? 6.110 A house may be kept warm by solar heating. Normally, water, with its high specific heat capacity, is used to store the heat. For example, a volume of 1500 gallons of water will store enough heat to keep a house warm overnight. Wood, on the other hand, is not as efficient for heat storage. A typical piece of wood has a density of 0.865 g/cm3 and a specific heat of 1.76 J/g °C. Wood is measured in board feet. A board foot is the volume of a piece of wood measuring exactly 12 inches × 12 inches × 1 inch. How many board feet of wood would be required to replace the 1500 gallons of water for heat storage? Hint: The heat capacity of the wood will equal the heat capacity of the water. 6.111 Hydrogen fluoride melts at –83 °C and boils at 20.0 °C. The enthalpy of fusion of hydrogen fluoride is 4.577 kJ/mol, and its enthalpy of vaporization is 748.5 kJ/mol. The specific heats of liquid and gaseous hydrogen fluoride are 0.148 J/g•K and 2.32 J/g•K, respectively. How much heat is required to convert 1350.0 g of solid hydrogen fluoride at the melting point to the vapor phase at 75 °C? 6.112 Methyl alcohol (CH3OH) melts at –98 °C and boils at 65 °C. The enthalpy of fusion of methyl alcohol is 3.17 kJ/mol, and its enthalpy of vaporization is 39.23 kJ/mol. The specific heats of liquid and gaseous methyl alcohol are 2.54 J/g•K and 1.37 J/g•K, respectively. How much heat is required to convert 250.0 g of solid methyl alcohol at the melting point to the vapor phase at 85 °C? 6.113 A sample of benzoic acid (HC7H5O2) weighing 0.286 g is burned in a bomb calorimeter (that is, at constant volume), raising the calorimeter temperature from 21.487 °C to 23.485 °C. A sample of caffeine (C 8H10N4O2) weighing 0.323 g was burned in the same calorimeter, and the temperature increased from 22.352 °C to 24.208 °C. The heat of combustion for benzoic acid is reported to be 3221.6 kJ/mol. Determine the heat of combustion, in kJ/mol, for caffeine. – 4.21 × 103 kJ/mol 6.114 Phosphorus pentachloride hydrolyzes in water according to the reaction PCl5(s) + 4 H2O(l) H3PO4(aq) + 5 HCl(aq) H° = –569 kJ From this reaction and Appendix C, estimate H°f for PCl5(s). 6.115 Chloroform (CHCl3) melts at –64 °C and boils at 62 °C. The enthalpy of fusion of chloroform is 8.798 kJ/mol, and its enthalpy of vaporization is 31.38 kJ/mol. The specific heats of liquid and gaseous chloroform are 0.967 J/g•K and 0.550 J/g•K, respectively. How much heat is required to convert 175.0 g of solid chloroform at the melting point to the vapor phase at 82 °C? 6.116 Isopropyl alcohol (C3H7OH) melts at –90.0 °C and boils at 82 °C. The enthalpy of fusion of isopropyl alcohol is 5.36 kJ/mol, and its enthalpy of vaporization is 42.11 kJ/mol. The specific heats of liquid and gaseous isopropyl alcohol are 2.68 J/g•K and 1.54 J/g•K, respectively. How much heat is required to convert 150.0 g of solid isopropyl alcohol at the melting point to the vapor phase at 122 °C? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 56 6.117 Liquid hydrazine (N2H4) and certain of its derivatives are used to fuel some rockets. Gaseous hydrazine burns with oxygen to produce nitrogen gas and water vapor. Hydrazine has a density of 1.004 g/mL, and it boils at 113.5 °C. The H°f for N2H4(g) is 95.40 kJ/mol. The heat of vaporization for N 2H4(l) is 44.77 kJ/mol. (a) How many kilojoules are produced when 1.00 gallon of liquid hydrazine are burned? (b) How many gallons of gasoline are required to produce an equal amount of energy? Assume gasoline is pure C8H18(l) (H°f = –249.9 kJ/mol) with a density of 0.7025 g/mL. 6.118 The gas constant, R, is useful for many gas law calculations. However, R is useful in many other situations. For gases, R often appears as 0.08206 L atm/mol K. In thermodynamics, it is more useful to use R in terms of energy (Joules). Convert 0.08206 L atm/mol K to J/mol K. 6.119 LP gas is liquid propane (C3H8). The substance vaporizes and burns as follows: C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(l) (a) The combustion of 20.0 g of propane requires how many grams of oxygen? (a) How many grams of carbon dioxide are produced when 150.0 cm3 of LP gas combusts? The density of LP gas is 0.5853 g/mL. (b) LP gas sometimes does not undergo complete combustion. When combustion is not complete carbon monoxide or carbon (soot) may form. Using the information in part (a), what is the percent yield if only 252.3 g of CO 2 form. (c) A 10.0-g sample of propane is placed in a bomb calorimeter with 25.0 g of oxygen gas. How many grams of water will form? 6.120 Natural gas is primarily methane (CH4). Methane burns with the oxygen in air to produce carbon dioxide gas and water vapor. The reaction is CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) (a) Calculate the maximum number of grams of carbon dioxide that may form from the reaction of 15.2 g of methane with 15.2 g of oxygen. (b) What is the percent yield if the mixture in part (a) only produces 9.75 g of carbon dioxide? Chapter 7 7.1 Corresponds to BLBMWS Section 6.1 7.1 Explain why electrons are the key to understanding the behavior of atoms. 7.2 What is the probe used to investigate atoms? 7.2 Corresponds to BLBMWS Section 6.1 7.3 Name three examples of radiant energy. 7.4 In addition to the speed of light, what are three other properties of light waves? 7.5 Draw a simplified wave and indicate the wavelength and the amplitude. 7.6 What is an angstrom? 7.7 Why is frequency often expressed as s–1 or 1/s? 7.8 Give the relationship between wavelength and frequency. 7.9 If the wavelength doubles, by what factor does the frequency change? 7.10 Which of the following could not possibly be correct wavelength units? (a) meters (b) centimeters (c) 1/meters (d) hertz (e) joules (f) angstroms (g) miles (h) s–1 (i) m/s (j) cm3 7.11 FM radio transmissions range from 87.5 to 108.0 MHz. Calculate the range of wavelengths this represents in centimeters. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 57 7.12 (a) Calculate the wavelength, in meters, of light with a frequency of 8.97 × 1012 s–1. (b) Calculate the frequency of light having a wavelength of 427 nm. (c) Calculate the frequency of light having a wavelength of 1.54 Å. (d) How many meters does a beam of light with a wavelength of 725 nm travel in 6.42 s? (e) How many seconds does it take a beam of light to travel 125 miles? 7.13 (a) A beam of light has a frequency of 3.55 × 10 13/s. What is the wavelength of this light in nanometers? (b) Calculate the frequency of a light wave with a wavelength of 0.500 pm. 7.16 (a) How long does it take a radio wave to travel 3000 miles across the United States? (b) How does the time necessary for a radio wave compare to the time necessary for a microwave to cross the United States? 7.17 The distance to Mars from the Earth varies from a minimum of 3.39 × 10 7 miles when both planets are on the same side of the Sun to a maximum of 2.49 × 10 8 miles when the two planets are on opposite sides of the Sun. (These values are upper and lower limits.) (a) How many seconds will it take for a radio message to reach a Mars lander on the Martian surface at the minimum distance? (b) How many seconds will it take a radio message to reach a Mars lander on the Martian surface at the maximum distance? 7.3 Corresponds to BLBMWS Sections 6.2-6.3 7.18 (a) Give the relationship between frequency and energy. (b) Give the relationship between energy and wavelength. 7.19 What did Planck call a unit of light energy? 7.20 What is a particle of light? 7.21 What does quantized mean? 7.22 Does the value of Planck's constant indicate that the typical photon will have a large or a small value? Explain. 7.23 You get an answer of 5.0 × 1019 J for a single photon of light. Explain why this is an unreasonable value. 7.24 What is the difference between an emission spectrum and an absorption spectrum? 7.25 The emission spectrum of sodium has a line with a wavelength of 589.0 nm. (a) What color is this light? (b) How does this wavelength compare to the wavelength of the corresponding transition in the absorption spectrum? 7.26 Calculate the energy of a photon of each of the following types of visible light. (a) red light at 685 nm (b) yellow light at 5920 Å (c) blue light at 0.468 m 7.27 What information in addition to the wavelength is necessary to calculate the energy of a photon of light? 7.28 (a) Calculate the frequency of a photon with 6.35 × 10 –19 J. (b) What is the energy of a photon with a frequency of 6.05 × 1012 s–1? (c) Calculate the energy of a photon with a wavelength of 13.2 m. (d) Calculate the wavelength of a photon with energy of 7.75 × 10 –19 J. 7.29 (a) Calculate the frequency of a photon with 1.37 × 10 –19 J. (b) What is the energy of a photon with a frequency of 8.25 × 1012 s–1? (c) Calculate the energy of a photon with a wavelength of 19.0 m. (d) Calculate the wavelength of a photon with energy of 8.75 × 10 –18 J. (a) 2.07 × 1014 s–1 (b) 5.47 × 10–21 J –20 –8 (c) 1.05 × 10 J (d) 2.27 × 10 m 7.30 Waves used for AM radio transmissions are between 530 and 1710 kHz. Determine the energy of each of these limiting values in joules. 7.31 Compare the energy of a photon with a wavelength of 652 nm to a photon with a wavelength of 775 Å. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 58 7.32 An X-ray diffractometer uses the diffraction of X-rays to determine the structures of molecules. One type of diffractometer uses X-rays generated from a copper target. These X-rays have a wavelength of 1.5418 Å. (a) Convert this wavelength to meters. (b) Determine the frequency of this radiation. (c) What is the energy of a photon of this radiation in joules? 7.33 Many organic compounds have a hydrogen atom attached to an oxygen atom. This arrangement absorbs infrared radiation with a wavelength near 3.0 m. How many joules does a photon of 3.0 m radiation supply? 7.34 Photons of sufficient energy striking a surface will cause electrons to be emitted by what is called the photoelectric effect. Excess energy from the photons is observed as kinetic energy of the emitted electrons. To drive an electron out of elemental cesium, a photon needs a minimum frequency of 5.17 × 10 14 s–1. (a) Calculate the energy of this photon. (b) Calculate the wavelength of this photon. (c) A sample of cesium metal is bombarded by photons with a wavelength of 475 nm. Calculate the maximum kinetic energy of the emitted electrons. (d) An electron weighs 9.109 × 10–28 g. What is the maximum velocity of the electrons emitted in part (c)? 7.35 For electrons to be emitted from platinum (Pt) metal through the photoelectric effect requires 545 kJ/mol (see Problem 7.34). (a) How much energy does each photon possess? (b) Determine the frequency of this light. (c) If X-rays with a wavelength of 0.154 nm are used, calculate the maximum kinetic energy of the electrons driven out of the platinum metal. 7.36 Electromagnetic radiation may be used to break the bonds between atoms. This process is known as photodissociation. To photodissociate carbon monoxide (CO) requires 1072 kJ/mol. Determine the wavelength of a photon of radiation with sufficient energy to break up a single carbon monoxide molecule. 7.37 A helium-neon laser, such as the one used in laser pointers, emits light with a wavelength of 6328 Å. (a) Calculate the wavelength of this light in meters. (b) Calculate the frequency of this radiation. (c) Calculate the energy of a photon of this radiation. 7.38 High-powered lasers emit exceedingly intense beams of high-energy light for very short periods. A highpowered laser emits a 75-ps pulse, which has a total energy of 25 joules. The wavelength emitted by the laser has a wavelength of 7500 Å. How many photons are in the pulse? 7.39 A particular type of photographic film is sensitive to infrared radiation with a maximum wavelength of 855 nm. (a) What is the frequency of 855-nm radiation? (b) How many kilojoules does a mole of 855-nm photons supply to the film? (a) 3.51 × 1014 s–1 (b) 1.40 × 102 kJ/mol 7.4 Corresponds to BLBMWS Section 6.3 7.40 What are the three postulates of the Bohr model of an atom? 7.41 (a) What type of spectrum results when an electron moves from the ground state to an excited state? (b) What type of spectrum results when an electron moves from an excited state to the ground state? 7.42 Why can there be more than one excited state but only one ground state? 7.43 Each of the following processes is accompanied by the gain or loss of energy. Decide if energy is gained or lost in each case. (a) An electron in the n = 3 shell of iron is completely removed from the atom. (b) An electron moves from the n = 2 to the n = 1 shell in a copper atom. (c) An electron moves from an orbital with a radius of 0.537 nm to one with a radius of 0.062 nm. 7.44 The spectrum of hydrogen corresponds to the movement of an electron from one energy level to another. However, the absorption of too much energy will remove the electron from the hydrogen atom instead of simply moving the electron between levels. To remove an electron from a ground-state hydrogen atom requires 1.31 MJ/mol. (a) What is the minimum frequency necessary to remove an electron? (b) What is the maximum wavelength necessary to remove an electron? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 59 7.5 Corresponds to BLBMWS Section6.4 7.45 What is the de Broglie relationship? 7.46 (a) What is the difference between v and ? (b) What are typical units for v and ? 7.47 What information is necessary to determine the wavelength of a particle? 7.48 (a) If two objects with different masses are moving at the same velocity, which will have the shorter wavelength? (b) If two objects with different velocities have the same mass, which will have the shorter wavelength? 7.49 Express a joule in terms of SI base units. 7.50 State the uncertainty principle. 7.51 What experiment led to the acceptance of the dual nature of electrons? 7.52 Why is it not possible to observe the wave nature of particles in our macroscopic world? 7.53 The de Broglie relationship may be used to determine the wavelength of any object. Determine the de Broglie wavelength of each of the following. (a) A 68-kg person sprinting at 10.0 km per hour (b) A 5.25 ounce baseball traveling at 95 mph (c) A helium atom traveling at 2500 mph. 7.54 The de Broglie relationship may be used to determine the wavelength of any object. Determine the de Broglie wavelength of each of the following. (a) A 125 pound person running at 1.20 miles per hour (b) A 2.00 ounce tennis ball traveling at 75 mph (c) A hydrogen molecule traveling at 1500 mph. 7.55 A transmission electron microscope may accelerate electrons to speeds nearly that of light. In a particular experiment, the electrons were found to have a de Broglie wavelength of 0.037 Å. How fast were these electrons moving (in m/s)? The mass of an electron is 9.109 × 10 –28 g. 7.6 Corresponds to BLBMWS Section 6.5 7.56 (a) What is the significance of in quantum mechanics? (b) What is the significance of 2? 7.57 How did Schrödinger's work alter Bohr's concept of electrons traveling in orbits? 7.58 What is an electron cloud? 7.7 Corresponds to BLBMWS Sections 6.5-6.6 7.60 List the names of the four quantum numbers. 7.61 (a) What is the rule governing the possible values of the quantum number n? (b) What is the rule governing the possible values of the quantum number l? (c) What is the rule governing the possible values of the quantum number ml? (d) What is the rule governing the possible values of the quantum number ms? 7.62 Each of the quantum numbers gives information on the features of an orbital or an electron. (a) What feature of an orbital does the principal quantum number, n, describe? (b) What feature of an orbital does the angular momentum quantum number, l, describe? (c) What feature of an orbital does the magnetic quantum number, ml, describe? (d) What feature of an electron does the electron-spin quantum number, ms, describe? 7.63 Define each of the following in your own words and tell how the quantum numbers relate to each. (a) shell (b) subshell (c) orbital (d) electron spin © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 60 7.64 Sketch a 2px, a 2py, and a 2pz orbital. Tell how these orbitals are alike and how they are different. 7.65 (a) Sketch a 1s, a 2s, and a 3s orbital. (b) Sketch a 2p, a 3p, and a 4p orbital. 7.66 Give the letter designation for each of the following values of l: 0, 1, 2, and 3. 7.67 (a) If the principal quantum number, n, is 4, what are the allowed values for the angular momentum quantum number, l? (b) If the angular momentum quantum number, l, has a value of 3, what are the allowed values for the magnetic quantum number, ml? (c) If the magnetic quantum number, ml, has a value of –2, what are the possible values for the electron spin quantum number, ms? 7.68 (a) List the allowed values for n, l, and ml for each electron in a 5d subshell. (b) List the allowed values for n, l, and ml for each electron in the third shell. 7.69 Which, if any, of the following would not be allowed: (a) 6s (b) 2d (c) 1p (d) 3d (e) 4f? 7.70 (a) How is a 4s orbital similar to a 3s orbital? (b) How is a 4s orbital different from a 3s orbital? (c) How is a 3s orbital similar to a 3p orbital? (d) How is a 3s orbital different from a 3p orbital? (e) How is a 3p x orbital similar to a 3pz orbital? (f) How is a 3px orbital different from a 3pz orbital? 7.71 For each of the following sets of quantum numbers give the appropriate subshell designation: n l (a) 3 0 (b) 4 3 (c) 5 1 (d) 3 2 (e) 1 0 7.72 The following sets of quantum numbers have been assigned to electrons: n l ml (a) 2 0 0 (b) 4 4 –1 (c) 5 3 –4 (d) 3 2 –2 (e) 1 2 0 If the combination is allowed, write the appropriate designation for the electron, e.g., 5d. If the combination is not allowed, make an appropriate correction, then give the appropriate designation. 7.8 Summary 7.73 Why is it possible to identify new elements based upon their spectra? 7.74 How does the calcium spectrum of a sample of calcium chloride compare to the calcium spectrum of a sample of calcium nitrate? 7.75 Why is it important for the Bunsen burner flame to be essentially invisible when used in spectroscopic analysis? 7.76 In addition to a line at 455.5 nm, the spectrum of cesium also has a line at 459.3 nm. (a) Determine the frequency in 1/s for the 459.3-nm line. (b) Determine the energy, in joules, for a photon of the 459.3-nm emission line. 7.77 Rubidium has lines in its absorption spectrum at 780.0 nm and 794.1 nm. (a) What color are the two lines? (b) What is the frequency, in s–1, for each of these lines? (c) What is the energy, in joules, for a photon of light for each of these lines? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 61 7.78 Scientists use atomic absorption spectroscopy to analyze various materials. The sample is vaporized by various means, and the amounts of light of specific wavelengths absorbed by the vapor indicate what elements are present in what amounts. The wavelengths that indicate the presence of the alkali and alkaline earth metals are Li 670.8 nm Be 234.9 nm Na 589.0 nm Mg 285.2 nm K 766.5 nm Ca 422.7 nm Rb 780.0 nm Sr 460.7 nm Cs 852.1 nm Ba 553.6 nm (a) For the elements absorbing light in the visible portion of the spectrum, determine the color of the radiation absorbed. (b) Rank these elements in order of increasing energy of the absorbed radiation. 7.79 The oxidizing agents in fireworks work because they release oxygen gas. Write balanced chemical equations for each of the following means of releasing oxygen gas. (a) Solid potassium perchlorate decomposes to form solid potassium chloride and oxygen gas. (b) Solid potassium chlorate decomposes to form solid potassium chloride and oxygen gas. (c) Solid potassium nitrate decomposes to form solid potassium nitrite and oxygen gas. 7.80 Gunpowder has charcoal, sulfur, and potassium nitrate present in a 3:2:15 ratio. One way of achieving this ratio is to mix 3.0 g of charcoal (C), 2.0 g of sulfur (S), and 15.0 g of potassium nitrate (KNO 3). These three substances react to produce carbon dioxide gas (CO2), sulfur dioxide gas (SO2), and solid potassium nitrite (KNO2). (a) Write a balanced chemical reaction for the combustion of gunpowder. (b) Which of the three components is the limiting reactant? (c) What is the total volume, in liters, of the gases produced if the temperature is 1500 °C at a pressure of 0.95 atm? (d) If the mixture in part (c) only produced 10.7 L of gas, what was the percent yield. 7.81 Potassium chlorate (KClO3) may substitute for the potassium nitrate in gunpowder. Unlike potassium nitrate, which only releases some of its oxygen to produce potassium nitrite, potassium chlorate decomposes to release all of its oxygen. How many grams of potassium chlorate are necessary to supply the same quantity of oxygen as 15.0 g of potassium nitrate? 7.82 Potassium perchlorate (KClO4) may substitute for the potassium nitrate in gunpowder. The decomposition of gunpowder containing potassium perchlorate produces carbon dioxide gas, sulfur dioxide gas, and solid potassium chloride. One way of balancing the equation is KClO4(s) + C(s) + S(s) KCl(s) + CO2(g) + SO2(g) (a) A gunpowder sample is prepared by mixing 6.0 g of charcoal (C) 4.0 g of sulfur (S) and 15.0 g of potassium perchlorate. Which of these is the limiting reagent? (b) Determine the partial pressure of each of the gases produced assuming the final temperature is 1250 °C at a pressure of 0.925 atm. (c) After the reaction in part (b), the sulfur dioxide was removed from the system and the pressure of the remaining gas was 0.455 atm. What was the percent yield of the reaction? 7.9 Summary 7.83 How many joules are there in a mole of photons with a frequency of 1.01 × 10 16 s–1? 7.84 Ultraviolet radiation is sometimes divided into three categories: UVA, UVB, and UVC. UVA consists of wavelengths between 320 and 400 nm, and is responsible for tanning of skin. UVB is responsible for sunburns, and has wavelengths ranging from 280 to 320 nm. UVC, which is almost entirely absorbed by the atmosphere, ranges from 200 to 290 nm. UVC is germicidal ultraviolet because even short exposure can be deadly to bacteria. (a) Determine the energy of a photon of UVA radiation with a wavelength of 365 nm. (b) Determine the energy of a photon of UVB radiation with a wavelength of 305 nm. (c) Determine the energy of a photon of UVC radiation with a wavelength of 245 nm. 7.85 The element cesium was discovered before scientists knew about electrons, protons, or neutrons in atoms. We now know that natural cesium consists entirely of cesium-132. How many protons, electrons, and neutrons are present in a cesium-132 atom? 7.86 Name or give the formula for each of the following compounds. (a) H 2S(g) (b) HF(g) (c) NH3(g) (d) CH4(g) (e) LiH (f) hydrogen bromide (g) hydrogen selenide (h) hydrobromic acid (i) calcium hydride (j) hydrofluoric acid © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 62 7.87 Name or give the formula for each of the following compounds. (a) XeF 2(s) (b) BrCl(g) (c) S2Cl2(s) (d) PBr3(l) (e) CI4(s) (f) disilicon hexachloride (g) nitrogen trifluoride (h) oxygen difluoride (i) iodine pentafluoride (j) diboron tetrachloride 7.88 The human eye requires at least 2.0 × 10–17 J for detection of light. How many photons of 660-nm green light are necessary to supply this amount of energy? 7.89 Name or give the formula for each of the following compounds. (a) lithium nitrate (b) magnesium phosphate (c) aluminum sulfate (d) barium fluoride (e) zinc acetate (f) SrH 2 (g) AlP (h) CdS (i) (NH4)HSO4 (j) NaHCO3 7.90 Name or give the formula for each of the following compounds. (a) calcium oxalate (b) potassium dichromate (c) zinc cyanide (d) aluminum cyanate (e) ammonium thiocyanate (f) Li2SO3 (g) CsMnO4 (h) (NH4)2HPO4 (i) BaCrO4 (j) SrCl2 7.91 The maximum amplitude of light from a typical incandescent light bulb has a wavelength of 550 nm. The light is about 5% of the total electrical energy passing through the bulb. How many photons does a 100-watt light produce in 1.0 minutes? 1 watt = 1 W = 1 J/s (exactly) 7.92 Provide the name or the formula for each of the following compounds: (a) FeCl 2 (b) ZnCl2 (c) FeCl3 (d) MnSO4 (e) CuF2 (f) lead(IV) oxide (g) tin(II) fluoride (h) manganese(III) oxide (i) gold(III) chloride (j) nickel(II) sulfide 7.93 Provide the name or formula for each of the following compounds: (a) iron(III) oxide (b) SnCl 4 (c) CuCN (d) tin(II) nitrate (e) PbI2 (f) nickel(II) sulfate (g) MnO2 (h) iron(II) carbonate (i) vanadium(V) oxide (j) Co 2O3 7.94 Most radioactive decay processes are accompanied by gamma rays. The energy of gamma radiation is typically given in megaelectronvolts (MeV a million electron volts). An electron volt (eV) is 1.602176 × 10 –19 J. The highest energy photon emitted by plutonium-239 has an energy of 0.41369 MeV. Determine the frequency of this photon in 1/s. 7.95 The highest energy gamma-ray photon emitted by americium-240 has an energy of 0.98764 MeV (see Problem 7.94 for the definition of this unit). Determine the wavelength of the photon in meters. 7.96 Barium compounds are often used to produce a bright green color in fireworks. Barium chlorate (Ba(ClO 3)2) one possible source of barium has the advantage that it can replace the potassium nitrate. Like most chlorates, barium chlorate decomposes to the metal chloride and oxygen gas. (a) Write a balanced equation for the reaction of solid carbon, sulfur, and barium chlorate to produce gaseous carbon dioxide and sulfur dioxide along with solid barium chloride. Assume there equal molar amounts of carbon and sulfur. (b) If you have 10.0 g of carbon, how many grams of each of the other solids can react? 7.97 A mixture of charcoal, sulfur, and potassium chlorate, all of which are solids, is used as a gunpowder substitute. This mixture will react to form gaseous carbon dioxide and sulfur dioxide along with solid potassium chloride. The following balanced chemical equation is illustrates one of the possible reaction mixtures. Determine the standard heat of reaction per mole of potassium chlorate. 3 C(s) + 3 S(s) + 4 KClO3(s) 3 CO2(g) + 3 SO2(g) + 4 KCl(s) 7.98 Provide the formula for each of the compounds in the following descriptions: (a) Heating calcium carbonate decomposes it to calcium oxide and carbon dioxide. (b) Hydrobromic acid reacts with zinc metal to form zinc bromide and hydrogen gas. (c) Copper(II) sulfate reacts with iron metal to form copper metal and iron(II) sulfate. (d) Potassium metal reacts with water to form potassium hydroxide and hydrogen gas. (e) Ammonium nitrate decomposes when heated to form dinitrogen oxide and water vapor. (f) Nitrogen gas reacts with hydrogen gas to form ammonia gas. (g) Copper metal reacts with concentrated nitric acid to form copper(II) nitrate, nitrogen oxide, and water. (h) Magnesium metal burns in oxygen gas to form magnesium oxide. (i) When heated in oxygen gas, tungsten metal forms yellow tungsten(VI) oxide. (j) Sodium carbonate reacts with hydrochloric acid to form sodium chloride, carbon dioxide, and water. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 63 7.99 Chlorophyll a absorbs sunlight to supply energy for photosynthesis. The maximum wavelength absorbed is 660 nm. The overall reaction of photosynthesis is 6 CO2(g) + 6 H2O(l) C6H12O6(s) + 6 O2(g) The carbohydrate formed is glucose (C6H12O6). (a) Determine the enthalpy change for this reaction in kilojoules per mole of glucose. (b) How many photons of 660-nm radiation are necessary to form a mole of glucose? 7.100 Ozone (O3) helps to protect the Earth's surface from high-energy photons of ultraviolet light. The energy from a photon of light can cause the following photodissociation process: O3(g) O2(g) + O(g) The standard heats formation of O3(g), O2(g), and O(g) are 143, 0.00, and 249 .2 kJ/mol, respectively. What is the longest wavelength of radiation that will photodissociate an ozone molecule? 7.101 How many microwave photons with a wavelength of 12.5 cm are needed to heat one cup of water from 25°C to boiling? Assume all the energy of the photons heats the water. The specific heat of water is 4.18 J/g•°C, the density of water is 1.00 g/cm3, and 1 cup = 236.3 mL 5.7 × 1028 photons 7.102 The names of certain compounds and ions may be very similar. Give the correct formula for each compound in the following pairs. (a) ammonium ion and ammonia (b) hydrochloric acid and chloric acid (c) lithium nitride and lithium nitrite (d) manganese(II) oxide and manganese(III) oxide (e) manganese(II) oxide and magnesium oxide (f) potassium nitrate and potassium nitrite (g) tungsten(IV) oxide and tungsten(VI) oxide (h) iodic acid and periodic acid (i) ammonium hydrogen phosphate and ammonium dihydrogen phosphate (j) calcium sulfite and calcium bisulfite 7.103 An alpha particle (mass = 6.6 × 10–24 g) emitted by radium travels at 3.4 × 107 mi/h. What is its de Broglie wavelength (in meters)? 6.6 × 10–15 m Chapter 8 8.1 Corresponds to BLBMWS Section 7.1 8.1 What are the underlying principles for the arrangement of elements in the periodic table? 8.2 When two atoms collide and react, what parts of the atom directly interact? 8.3 What is the rule that applies to the possible values of each of the four quantum numbers? 8.4 Review the preceding chapter and explain what each of the four quantum numbers indicates about the behavior of an electron in an atom. 8.5 If the value of the principal quantum number, n, is 4, what are the possible values of the l quantum number? 8.6 What are the possible values of the ml quantum number if n = 3? 8.7 What are the possible values of the ml quantum number if l = 2? 8.8 What are the possible values of the ms quantum number if l = 2? 8.2 Corresponds to BLBMWS Sections 6.7 and 7.2 8.9 In your own words, state the Pauli exclusion principle. 8.10 What is the relationship between two spin-paired electrons? 8.11 Which possesses the lowest energy, the excited state or the ground state? Explain your answer. 8.12 How does the German meaning of "aufbau" apply to how the electrons fill the orbitals? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 64 8.13 What are the first ten orbitals to fill in an atom? List these orbitals in order of increasing energy. 8.3 Corresponds to BLBMWS Sections 6.7-6.8 8.14 How does an orbital diagram differ from an electron configuration? 8.15 What is the first element to which Hund's rule becomes applicable? Why is this true? 8.16 The following sets of quantum numbers have been assigned to electrons: n l ml (a) 2 0 0 (b) 4 4 –1 (c) 5 3 –4 (d) 3 2 –2 (e) 1 2 0 If the combination is allowed, write the appropriate designation for the electron, e.g., 5d. If the combination is not allowed, make an appropriate correction, then give the appropriate designation. 8.17 Determine the maximum number of electrons allowed to have the following quantum numbers in a single atom: (a) n = 4 (b) n = 3 and l = 2 (c) n = 6, l = 1, and ml = 0 (d) n = 5, l = 0, ml = 0, and ms = +1/2. 8.18 List all possible sets of the four quantum numbers for a 2p electron on a hydrogen atom. n l ml ms 2 1 –1 –1/2 2 1 0 –1/2 2 1 +1 –1/2 2 1 –1 +1/2 2 1 0 +1/2 2 1 +1 +1/2 8.19 List all possible sets of the four quantum numbers for a 3d electron on a hydrogen atom. 8.20 How do valence electrons differ from core electrons? 8.21 The electron configuration of a potassium atom is 1s 22s22p63s23p64s1. (a) How many core electrons does a potassium atom have? (b) How many valence electrons does a potassium atom have? 8.22 Give the complete electron configuration for each of the following atoms: (a) O (b) Mn (c) Yb (d) Os (e) Cu (a) 1s22s22p4 (b) 1s22s22p63s23p64s23d5 (c) 1s22s22p63s23p64s23d104p65s24d105p66s24f145d1 (d) 1s22s22p63s23p64s23d104p65s24d105p66s24f145d6 (e) 1s22s22p63s23p64s13d10 8.23 Draw orbital diagrams showing the electrons in the outside shell of each of the following atoms: (a) C (b) Al (c) As (d) Ba (e) Xe. 8.24 (a) List in order of increasing energy the various subshells of the fourth shell. (b) List in order of increasing energy the orbitals in the 4p subshell. 8.25 Which element is indicated by each of the following electron configurations? (a) 1s22s22p5 (b) 1s22s22p63s23p64s23d7 (c) 1s22s22p63s23p64s23d104p65s1 (d) 1s22s22p63s23p64s23d104p65s24d105p66s24f8 (e) 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p4 © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 65 8.26 Convert each of the following excited-state electron configurations to ground-state electron configurations, and identify the element. (a) 1s22s12p6 (b) 1s22s22p63s23p54s1 (c) 1s22s22p63s23p64s13d104p4 (d) 1s22s22p63s23p64s23d104p65s24d95p2 (e) 1s22s22p63s23p64s23d104p65s24d105p66s24f135d8 8.27 (a) What first-row transition elements have electron configurations that are exceptions to the aufbau principle? (b) Give the electron configuration of the first-row transition elements that are exceptions to the aufbau principle. 8.28 (a) Define the terms paramagnetic and diamagnetic. (b) Give the names of two second-period elements whose atoms are paramagnetic. (c) Give the names of two second-period elements with atoms that are diamagnetic. 8.29 What experimental evidence shows that a substance is paramagnetic? 8.30 For determining if an atom is paramagnetic, which is more useful, an electron configuration or an orbital diagram? 8.31 Which of the following atoms is the most paramagnetic? N, Ar, Mn, K, or O 8.32 How many elements in the fourth period on the periodic table are diamagnetic in the ground state? 8.33 (a) Draw an orbital diagram for the outer shell of a magnesium atom. (b) Draw an orbital diagram for the outer shell of a phosphorus atom. (c) Describe each of these two elements as paramagnetic or diamagnetic. 8.4 Corresponds to BLBMWS Section 6.9 8.34 Sketch a periodic table. (a) On your sketch, indicate where the s orbitals are filling. (b) On your sketch, indicate where the p orbitals are filling. (c) On your sketch, indicate where the d orbitals are filling. (d) On your sketch, indicate where the f orbitals are filling. (e) On your sketch, indicate where the 5f orbitals are filling. 8.35 What distinguishes the electron configuration of helium from that of all the other noble gases? 8.36 Based on the position of each of the following elements on the periodic table, give the outer-shell electron configuration of each: (a) N (b) Cl (c) Ge (d) Sr (e) Kr. 8.37 Which outermost orbitals are being filled in the following types of elements? (a) representative elements (b) transition metals (c) inner transition metals 8.38 In terms of orbital filling, what distinguishes the lanthanide series from the actinide series? 8.39 List the similarities and differences between the electron configurations of nitrogen (N), phosphorus (P), and bismuth (Bi). 8.40 Some properties for the elements silicon (Si), and tin (Sn) are listed below. Mendeleev used the properties of these two elements to predict the properties of eka-silicon (later discovered and named germanium). (a) Predict the corresponding properties for germanium (Ge). (b) Use the CRC Handbook of Chemistry and Physics to determine the correct answers for these properties. Property Silicon Germanium Tin (a) predicted (b) CRC Atomic weight 28.08 amu _____ _____ 118.71 amu Formula of oxide SiO2 _____ _____ SnO2 and SnO Density of element 2.32 g/cm3 _____ _____ 5.75 g/cm3 Melting point of element 1410°C _____ _____ 232 °C Boiling point of element 2355°C _____ _____ 2270 °C © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 66 8.41 Some properties for the elements potassium (K) and cesium (Cs) are listed below. (a) Predict the corresponding properties for rubidium (Rb). (b) Use the CRC Handbook of Chemistry and Physics to determine the correct answers for these properties. Property Potassium Rubidium Cesium (a) predicted (b) CRC Atomic weight 39.098 amu _____ _____ 132.905 amu Formula of chloride KCl _____ _____ CsCl Density of element 0.86 g/cm3 _____ _____ 1.93 g/cm3 Melting point of element 63.25°C _____ _____ 28.5 °C Boiling point of element 760°C _____ _____ 671 °C 8.5 Corresponds to BLBMWS Section 7.3 8.42 Why do the atomic radii tend to increase down a column on the periodic table? 8.43 (a) Why is an argon atom smaller than a krypton atom? (b) Why is an argon atom smaller than a chlorine atom? 8.44 Describe why the outer shell of the noble gas neon has a smaller radius than the outer shell of nitrogen. 8.45 Using only the periodic table, arrange the elements in each of the following groups in order of decreasing atomic radius: (a) C, S, Ca, Pt, and Sb (b) Cr, Ga, N, Ta, and Mo (c) Al, Te, He, Mg, and Cs. 8.46 What is "effective nuclear charge," and why is it more important than the nuclear charge of an atom? 8.47 Why does the effective nuclear charge increase across a period? 8.48 How does the effective nuclear charge affect the atomic radius of an atom? 8.49 Which of the following isotopes has the highest effective nuclear charge? choice. 23 Na, 25Na, or 24Mg Explain your 8.6 Corresponds to BLBMWS Section 7.4 8.50 What is the definition of ionization energy? 8.51 Why do the successive ionization energies of an element increase? 8.52 Write balanced chemical equations illustrating the first and second ionization energies of calcium. 8.53 Compare the second ionization energies of the elements K and Ca. Why is the value of potassium larger than that of calcium? 8.54 Using only the periodic table, pick the member of each pair with the higher ionization energy: (a) Na and Rb (b) Si and Sb (c) Li and Be (d) B and Al (e) C and N. 8.55 For each of the following pairs, indicate which element has the larger first ionization energy. In each case, provide an explanation in terms of electron configuration and effective nuclear charge. (a) P, Cl (b) Al, Ga (c) Cs, La (d) Si, N. 8.56 (a) Write electron configurations for Ca and Ca2+. (b) Write the electron configurations for Fe, Fe2+, and Fe3+. (c) Use your answers to parts (a) and (b) to illustrate why the meaning of valence electrons is different for the representative elements and the transition metals. 8.57 Write the full electron configuration of each of the following atoms or ions. Co, Co2+, and Co3+ 8.58 Why are cations smaller than their parent atom? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 67 8.59 The following trends in the sizes of atoms or ions have been observed. Explain the trend in each case. (a) Li + < K+ < Cs+ (b) S2+ < S < S2– (c) Cr6+ < Cr3+ < Cr2+ < Cr 8.60 The following trends in the sizes of atoms or ions have been observed. Explain the trend in each case. (a) La 3+ < Ba2+ < Cs+ (b) F– < O2– < N3– (c) K+ < Ar < Cl– < S2– 8.61 List the atoms or ions in each set in order of decreasing radius: (a) Y 3+, Rb+, and Sr2+ (b) Ne, F–, and Na+ (c) P3–, Cl–, and S2– (d) Li+, Ca2+, and Cl– (e) Kr, Br, and Br– (a) Rb+ > Sr2+ > Y3+ (b) F– Ne > Ne+ (c) P3– > S2– > Cl– (d) Cl– > Ca2+ > Li+ (e) Br– > Br > Kr 8.7 Corresponds to BLBMWS Section 7.5 8.62 What is the definition of electron affinity? 8.63 Write balanced chemical equations illustrating the first and second electron affinities of sulfur. 8.64 Why, in most cases, is it more difficult to form a dianion (–2 ion), than it is to form a –1 ion? 8.65 Why are anions larger than their parent atom? 8.66 Write the full electron configuration of each of the following atoms or ions. O, O 2–, and Ne 8.67 (a) Why are the electron affinities of the noble gases negligible? (b) Why are the electron affinities of the alkaline earth metals negligible? 8.68 Using electron configurations show why it is easier to add an electron to a potassium atom than to a calcium atom. 8.69 (a) Write the electron configuration of oxygen. (b) Write electron configurations for the anions formed as electrons are added to an oxygen atom. (c) Why is the addition of two electrons the limit for an oxygen atom? (d) Based on your answer to part (c) what is the limit for a nitrogen atom? 8.8 Corresponds to BLBMWS Section 8.4 8.70 What is electronegativity? 8.71 (a) Which of the representative elements has the highest electronegativity? (b) Which of the representative elements has the lowest electronegativity? 8.72 Draw a simplified sketch of the periodic table and indicate the general trend(s) for electronegativity. 8.73 (a) What type of ions do atoms with high electronegativities tend to form? (b) What type of ions do atoms with low electronegativities tend to form? 8.74 (a) What is the general trend in electronegativity moving to the right on the periodic table? (b) What is the general trend in electronegativity moving from top to bottom on the periodic table? 8.75 (a) How does the trend in electronegativities compare to the trend in ionization energies? (b) How does the trend in electronegativities compare to the trend in electron affinities? 8.76 Using only the periodic table as a guide, arrange the elements in each of the following sets in order of increasing electronegativity. (a) F, O, Cl (b) Br, Cl, I (c) H, B, C (d) Li, K, Cs (e) Al, Na, P (a) Cl < O < F (b) I < Br < Cl (c) B < H < C (d) Cs < K < Li (e) Na < Al < P 8.77 Using only the periodic table, pick the member of each pair with the higher ionization energy: (a) Na and Rb (b) Si and Sb (c) Li and Be (d) B and Al (e) C and N. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 68 8.9 Corresponds to BLBMWS Sections 7.6-7.8 8.78 Which element is considered the "unique" element? Why is this element unique? 8.79 Sketch a simplified periodic table and illustrate the trend(s) in metallic character. 8.80 (a) In general, how do the properties of metals compare to the properties of nonmetals? (b) How do the properties of metalloids compare to metals and nonmetals? 8.81 Use the periodic table to pick the more metallic member of each pair: (a) C and Pb (b) Li and O (c) Ca and Ba (d) Se and Po (e) Br and I. (a) Pb (b) Li (c) Ba (d) Po (e) I 8.82 Arrange the following elements in order of increasing electrical conductivity. Si, K, P 8.83 (a) Why is barium more reactive than magnesium? (b) Why is barium less reactive than cesium? 8.84 Most oxides may be described as being either acidic or basic. Arrange the following oxides from the most acidic to the most basic: calcium oxide (CaO), cesium oxide (Cs2O), sulfur dioxide (SO2), zinc oxide (ZnO), silicon dioxide (SiO2), dichlorine heptoxide (Cl2O7), thallium(III) oxide (Tl2O3), and sulfur trioxide (SO3) 8.85 (a) What type of compounds do nonmetal oxides tend to form when added to water? (b) What type of compounds do metal oxides tend to form when added to water? 8.86 (a) What type of ions do metals tend to form? (b) What type of ions do nonmetals tend to form? (c) What happens when a typical metal oxide reacts with a typical nonmetal oxide? 8.87 Complete and balance the equations for each of the following: (a) calcium oxide reacts with water (b) sulfur dioxide reacts with water (c) manganese(II) oxide reacts with hydrochloric acid (d) carbon dioxide reacts with an aqueous solution of sodium hydroxide (e) calcium oxide reacts with sulfur dioxide. 8.88 Complete and balance the equations for each of the following: (a) tetraphosphorus decaoxide dissolves in water (b) chromium(III) oxide reacts with nitric acid (c) dinitrogen pentoxide reacts with water (d) nickel(II) oxide reacts with sulfuric acid (e) cobalt(II) oxide reacts with sulfur trioxide. 8.89 Complete and balance the equations for each of the following reactions. (a) Solid selenium reacts with calcium metal. (b) Dinitrogen oxide decomposes to the elements. (c) Magnesium metal burns in oxygen gas. (d) Sulfur vapor reacts with beryllium metal. (e) Water vapor burns in fluorine gas to produce hydrogen fluoride gas and oxygen gas. 8.90 Complete and balance the equations for each of the following reactions. (a) Liquid bromine reacts with calcium metal. (b) Carbon burns in air. (c) Xenon gas reacts with fluorine gas. (d) Lithium metal reacts with oxygen gas. (e) Aluminum metal reacts with carbon. 8.91 Complete and balance the equations for each of the following: (a) sodium is placed in a beaker of water (b) calcium is placed in a beaker of water (c) lithium metal reacts with liquid bromine (d) hydrogen gas burns in fluorine gas (e) magnesium metal reacts when heated with nitrogen gas. 8.92 Complete and balance the equations for each of the following: (a) calcium metal reacts with chlorine gas (b) lithium metal reacts with hydrogen gas (c) barium metal reacts with water (d) beryllium metal reacts with chlorine gas (e) potassium oxide reacts with water. 8.93 Hydrogen may form two ions. Give the electron configuration of each of these ions. 8.94 (a) Define a covalent hydride and give an example. (b) Define an ionic hydride and give an example. (c) Define a metallic hydride and give an example. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 69 8.95 Which alkali metals are essential to living organisms? 8.96 The alkali metals are very reactive. Write a general chemical equation to illustrate the reaction of an alkali metal with water. 8.97 Write balanced chemical equations for the reaction of each of the alkali metals with oxygen. 8.98 Which alkaline earth metals are among the most abundant elements in the Earth's crust? 8.99 How does beryllium hydroxide differ from barium hydroxide? 8.100 How does the most stable thallium ion differ from the most stable aluminum ion? 8.101 How is aluminum hydroxide different from the alkali metal hydroxides? 8.102 (a) What is an allotrope? (b) Describe the structures of the following allotropes of carbon: diamond, graphite, and buckminsterfullerene. 8.103 Which carbon family element is the most abundant element in the Earth’s crust? 8.104 What are the highest and lowest oxidation numbers for the elements in the nitrogen family? 8.105 Which elements in the nitrogen family are essential to life? 8.106 Which oxygen-containing anion is analogous to the disulfide ion? 8.107 (a) What is the formula for a sulfur molecule? (b) What is the formula of ozone? (c) How do these formulas differ from that of a "normal" oxygen molecule? 8.108 Which elements in the oxygen family are essential to most living organisms? 8.109 Which halogen forms only one type of ion in its compounds? 8.110 The chapter identifies the following as a disproportionation reaction: Cl2(g) + H2O(l) HCl(aq) + HClO(aq) (a) Assign oxidation numbers to each of the elements in this equation. (b) Which element undergoes oxidation? (c) Which element undergoes reduction? (d) The answers to parts (b) and (c) provide a clue to the definition of a disproportionation reaction. What do you think is the definition of a disproportionation reaction? 8.111 How many compounds are known to contain simple noble gas ions? 8.112 (a) What is the formula for platinum(VI) fluoride? (b) Why is platinum(VI) fluoride important to the chemistry of the noble gases? 8.113 (a) List five of the major biologically important elements. (b) List five of the biologically important trace elements. 8.114 (a) Which elements are important to biological systems because they form stable ions? (b) Some elements are biologically important because they participate in oxidation-reduction reactions. Where are most of these ions located on the periodic table? 8.10 Summary 8.115 (a) When Mendeleev first developed the periodic table, he arranged the elements in order of increasing atomic weight. What two pairs of representative elements are exceptions to this arrangement? (b) Which of these two pairs did not matter to Mendeleev? Why? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 70 8.116 The most abundant isotopes of the nitrogen family elements are 14N, 31P, 75As, 121Sb, and 209Bi. List the number of protons, neutrons, and electrons in an atom for each of these isotopes. 8.117 Label each compound from the following list as a solid or a gas based upon its most likely physical state at room temperature. Explain your choice in each case. (a) hydrogen chloride (HCl) (b) sodium chloride (NaCl) (c) strontium oxide (SrO) (d) zinc fluoride (ZnF2) (e) dinitrogen oxide (N2O) (f) hydrogen sulfide (H2S) (g) carbon monoxide (CO) (h) sulfur dioxide (SO2) (i) aluminum sulfide (Al2S3) (j) ammonium nitrate (NH4NO3) 8.118 Locate the following elements on the periodic table: N, Te, Ge, Pb, and Cl. (a) Arrange these elements in order of decreasing electron affinity. (b) Arrange these elements in order of decreasing first ionization energy. (c) Arrange these elements in order of decreasing atomic radius. (a) Cl > N > Ge > Te > Pb (b) Cl > N > Ge > Te > Pb (c) Pb > Te > Ge > Cl > N 8.119 The following table gives the successive ionization energies for carbon and silicon. All values are in MJ/mol. First Second Third Fourth Fifth C 1.1 2.4 4.6 6.2 37.8 Si 0.8 1.6 3.2 4.4 16.1 (a) Compare any of the values for carbon with the corresponding value for silicon. Why is the silicon value lower? (b) Why do both elements show a similar trend of increasing values? (c) Why is there an apparent break in the trend between the fourth and fifth ionization energies? 8.120 Explain any similarities and differences between the elements potassium and calcium with respect to (a) size of the atoms (b) expected ionic charge (c) electron configuration (d) ionization energy (e) behavior with water. 8.121 Write complete, balanced equations for the following pairs of reactions. Compare the products of the reactions in each pair. (a) Potassium metal reacts with iodine vapor, and hydrogen gas reacts with iodine vapor. (b) Strontium metal reacts with chlorine gas, and strontium metal reacts with hydrogen gas. 8.122 The synthesis of sulfuric acid begins with the combustion of sulfur to produce sulfur dioxide. In the presence of a catalyst, oxygen oxidizes the sulfur dioxide to sulfur trioxide. Dissolving the sulfur trioxide in sulfuric acid produces disulfuric acid (H2S2O7). Finally, adding water to disulfuric acid produces sulfuric acid. (a) Write balanced chemical equations for each step in the synthesis of sulfuric acid. (b) Beginning with 1.00 ton (2000.0 pounds) of sulfur, how many gallons of sulfuric acid may be produced? (c) The density of sulfuric acid is 1.84 g/mL. What is the total volume of air required for the oxidation of 1.00 ton of sulfur in the preparation of sulfuric acid? The mole fraction of oxygen in air is 0.20, and the air is at 27°C at a pressure of 742 mmHg. 8.123 Give the full electron configuration of each of the following. (a) Silicon (b) Iron (c) Lead 8.124 A 2.50 g sample of sodium reacted completely with water. How many milliliters of dry H2 evolved at 21°C and 748 mmHg. The reaction is: 2 Na(s) + 2 H2O(l) 2 NaOH(aq) + H2(g) 8.125 Using only the periodic table as a guide arrange each of the following sets of elements in order of decreasing electronegativity. (a) Br, I, and Cl (b) Mg, Al, and Cs (c) C, N, and Br (d) Se, B, and F (e) H, C, and Na. Chapter 9 9.1 Which electrons are the key to the chemistry of the atoms? 9.2 Where are the valence electrons of the representative elements located? 9.3 If zinc is treated as a representative element, how many valence electrons does it have? 9.4 How many valence electrons does each of the following atoms possess? (a) Na (b) Al (c) As (d) Se (e) F © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 71 9.2 Introduction to Chapter 8 in BLBMWS 9.5 What are the three bonding types? 9.6 What type of bonding occurs in each of the following? (a) steel, (b) table salt, (c) water 9.7 In which of the following is the bonding similar to that found in water? (a) gasoline, (b) chalk, (c) brass, (d) vegetable oil, (e) sugar 9.8 In which of the following substances is the bonding similar to that found in table salt? (a) bronze, (b) chalk, (c) sugar, (d) rubbing alcohol, (e) wood ash 9.9 If the only information you have concerning a substance is its composition, how can you predict if the substance has ionic, covalent, or metallic bonding? 9.10 What would be a better method than simply examining the composition to predict the type of bonding present in a substance? 9.11 (a) If a compound is ionic, what is the minimum electronegativity difference between the atoms? (b) If a compound is covalent, what is the minimum electronegativity difference between the atoms? 9.12 If two atoms have an electronegativity difference of 1.0, what is the best description of the type of bonding present? 9.13 Which element presents many exceptions to bonding predictions? 9.14 Arrange the following compounds in order of increasing ionic bonding character. (a) MgCl 2 (b) SiCl4 (c) NaCl (d) AlCl3 (e) PCl5 PCl5 < SiCl4 < AlCl3 < MgCl2 < NaCl 9.3 Corresponds to BLBMWS Section 8.1 9.15 In your own words, define a Lewis symbol. 9.16 Show four different ways to draw the Lewis symbol of a hydrogen atom. 9.17 Draw the Lewis symbol of each element in the third period on the periodic table. 9.18 Draw Lewis symbols for each of the following. (a) As (b) As 3– (c) Ba2+ (d) O– (e) Pb2+ 9.19 Draw Lewis symbols for each of the following. (a) Si (b) Kr (c) P2– (d) Sb3+ (e) Cd 9.20 Draw a Lewis symbol for each of the following: (a) Li (b) Se (c) Pb (d) Al 3+ (e) P3– 9.21 (a) Which elements are most likely to form anions? (b) Which elements are most likely to form cations? (c) Locate these groups on the periodic table. (a) Nonmetals (b) Metals (c) Nonmetals are to the (upper) right and metals are to the left 9.22 Why are cations like F7+ and S6+ unlikely to form? 9.23 Why are anions like B5– and Mg6– unlikely to form? 9.24 The electron configuration for sulfur is 1s22s22p63s23p4. (a) How many valence electrons does sulfur have? (b) What is the relationship between sulfur's valence electrons and its position on the periodic table? (c) Which of the electrons on sulfur are the valence electrons? (d) How many electrons would sulfur be expected to gain when forming an anion? (e) Write the electron configuration for the anion sulfur is expected to form. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 72 9.25 Polonium (Po) is in the oxygen family on the periodic table. What type of ion or ions would you expect it to form? Explain. 9.26 Write balanced chemical equations for each of the following properties. Give the Lewis symbols for all reactants and products. (a) the first electron affinity of phosphorus (b) the second electron affinity of phosphorus (c) the third electron affinity of phosphorus 9.27 Give Lewis structures for the following compounds and identify any ions that do not obey the octet rule. (a) calcium fluoride (b) lithium phosphide (c) tin(II) oxide (d) sodium sulfide (e) magnesium nitride 9.4 Corresponds to BLBMWS Section 8.2 9.28 (a) When forming a stable anion, what is the maximum number of electrons an atom may gain? (b) When forming a stable cation, what is the maximum number of electrons an atom may lose? 9.29 (a) Is the formation of a cation oxidation or reduction? (b) Is the formation of an anion oxidation or reduction? 9.30 What is the source of electrons for the formation of an anion? 9.31 (a) Draw the Lewis symbols of a nitrogen atom, an oxygen atom, and a fluorine atom. (b) On your Lewis symbols, indicate where the atom will gain electrons to form an anion. 9.32 What complication occurs in predicting the cations that the lower p-block elements may form? 9.33 Diagram the reaction of potassium atoms with bromine atoms to form a compound using Lewis symbols. 9.34 Diagram the reaction of calcium atoms with fluorine atoms using Lewis symbols. 9.35 Each of the following pairs of elements will form an ionic compound. In each case, predict the formula for the compound. (a) Li and I (b) Ba and Cl (c) Na and P (d) Ca and Se (e) Al and S (a) LiI (b) BaCl2 (c) Na3P (d) CaSe (e) Al2S3 9.36 Each of the following pairs of elements will form an ionic compound. In each case, predict the formula for the compound. (a) Na and F (b) Ca and Br (c) Li and N (d) Mg and S (e) Al and Se 9.37 What is the name or the formula of each of the following compounds: (a) FeCl3 (b) CdCl2 (c) MnCl3 (d) CoSO4 (e) ZnF2 (f) manganese(IV) oxide (g) lead(II) fluoride (h) iron(III) oxide (i) silver(II) fluoride (j) nickel(II) thiosulfate 9.38 What is the name or the formula of each of the following compounds: (a) chromium(III) oxide (b) VCl4 (c) CuI (d) iron(II) nitrate (e) PbBr2 (f) titanium(III) sulfate (g) CrO2 (h) iron(III) phosphate (i) chromium(VI) oxide (j) Mn2O3 9.5 Corresponds to BLBMWS Section 8.1 9.39 State the octet rule in your own terms. 9.40 Why would it be possible to change the “octet rule” to the “noble gas rule”? 9.41 Using electron configurations, to show why each of the following obeys the octet rule. (a) O 2– (b) K+ (c) Xe (d) As3– (e) Ra2+ 9.42 Does the hydrogen ion obey the octet rule? Does the hydride ion? 9.43 Assign the most likely charge to the ions formed by the second period elements. Which of these ions do and which do not obey the octet rule? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 73 9.44 What amount of energy change is necessary to convert a potassium atom into an ion obeying the octet rule? What is the name of this energy change? 9.45 Write a chemical equation illustrating the reaction of a chlorine atom to produce an ion that obeying the octet rule. What is the name of this energy change? 9.46 Which of the following ions obey the octet rule? (a) O2– (b) Li+ (c) Fe2+ (d) Ba2+ (e) P3– (a), (d), and (e) follow the octet rule 9.47 Which noble gas does not obey the octet rule? 9.6 Corresponds to BLBMWS Section 8.2 9.48 Energy is required to produce a cation, and energy is often required to produce an anion. What is the source of this energy? 9.49 In your own words, define lattice energy. Is the lattice energy always endothermic or always exothermic? 9.50 The Born-Haber cycle requires the following information (a) the standard heat of formation, (b) the ionization energy, (c) the electron affinity, (d) the sublimation energy, (e) and the bond energy. Define each of these terms. 9.51 The construction of a Born-Haber is made possible by what chemical law? 9.52 If the Born-Haber cycle shown in section on the Born-Haber cycle were for magnesium bromide (MgBr 2) what values would you need to change? What additional value(s) would be necessary? 9.53 Which member of each of the following pairs has the higher lattice energy? Explain your answer in each case. (a) NaCl and KCl (b) BaCl2 and MgCl2 (c) NaF and CaO 9.54 Aluminum oxide (Al2O3) is a very hard material with an extremely high melting point. Explain these properties in terms of its expected lattice energy. 9.55 Determine the lattice energy for potassium fluoride. Use the following information: heat of formation of potassium fluoride, −568.6 kJ/mol; ionization energy of potassium, 419 kJ/mol; electron affinity of fluorine, −328 kJ/mol; sublimation energy of potassium, 89.2 kJ/mol; bond energy of fluorine, 150.6 kJ/mol. 9.56 Determine the lattice energy for calcium oxide. Use the following information: heat of formation of calcium oxide, −635.1 kJ/mol; first ionization energy of calcium, 0.5898 MJ/mol; second ionization energy of calcium, 1.1454 MJ/mol; first electron affinity of oxygen, −141 kJ/mol; second electron affinity of oxygen, 744 kJ/mol; sublimation energy of calcium, 192.6 kJ/mol; bond energy of oxygen, 498.7 kJ/mol. 9.57 Determine the ionization energy for cesium. Use the following information: lattice energy for cesium fluoride (CsF), 743.9 kJ/mol; heat of formation of cesium fluoride, −554.7 kJ/mol; electron affinity of fluorine, −328 kJ/mol; sublimation energy of cesium, 76.7 kJ/mol; bond energy of fluorine, 150.6 kJ/mol. 9.58 Determine the electron affinity for bromine. Use the following information: lattice energy for rubidium bromide (RbBr), 654.0 kJ/mol; heat of formation of rubidium bromide, −389.2 kJ/mol; ionization energy of rubidium, 403 kJ/mol; sublimation energy of rubidium, 85.81 kJ/mol; bond energy of bromine, 192.5 kJ/mo; heat of vaporization for bromine, 30.91 kJ/mol. 9.59 If the compound NaF2 existed, its lattice energy would be 2180 kJ/mol. Determine the heat of formation of NaF2. In view of the heat of formation for NaF2, would you expect this compound to exist? Use the following additional information: electron affinity of fluorine, −328 kJ/mol; sublimation energy of sodium, 107.76 kJ/mol; bond energy of fluorine, 150.6 kJ/mol; first ionization energy of sodium, 496 kJ/mol; second ionization energy of sodium, 4562 kJ/mol. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 74 9.7 Summary 9.66 An adult has about 4.7 L of blood plasma. (a) What is the minimum percentage of the body's potassium in the blood plasma? (b) What is the maximum percentage of the body's potassium in the blood plasma? 9.67 If a person has an iron level of 0.02 mmol/L, how many milligrams of iron(II) sulfate (FeSO4) are necessary to raise this to 180 mg/kg of body weight? Assume there are 4.7 L of blood plasma present, and the person weighs 73 kg. 9.68 List the three solubility rules. 9.69 What are the common exceptions to Solubility Rule 1? 9.70 What is the relationship between the solubility rules and the lattice energy? 9.71 You are given an ionic compound; the cation has a +1 charge, and the anion has a –1 charge. The compound is insoluble in water. What does this tell you about the relative electronegativities of the elements in the compound? 9.72 What effect does the size of an ion have on the solubility of compounds containing the ion? 9.73 Explain why lithium fluoride (LiF) is expected to be less soluble than cesium iodide (CsI). 9.74 When a hydrosulfuric acid solution and an iron(II) sulfate solution mix, iron(II) sulfide precipitates. (a) Write a balanced molecular equation for this reaction. (b) Write a net ionic equation for this reaction. (c) Which solubility rule is applicable? (d) If you began with 100.0 mL of 0.10 M hydrosulfuric acid and 175.0 mL of 0.50 M iron(II) sulfate solution, how many grams of unreacted hydrosulfuric acid are present in the solution? (e) What is the molarity of unreacted hydrosulfuric acid in part (d)? 9.75 Write net ionic equations for each of the following reactions. (a) An aqueous solution of ammonium iodide (NH4I) reacts with an aqueous solution of lead(II) nitrate (Pb(NO 3)2). (b) An aqueous solution of potassium sulfide (K2S) reacts with an aqueous solution of cobalt(II) sulfate, (CoSO4). (c) An aqueous solution of ammonium arsenate ((NH4)3AsO4) reacts with an aqueous solution of magnesium chlorate (Mg(ClO 3)2). (d) An aqueous solution of barium nitrite (Ba(NO2)2), reacts with an aqueous solution of lithium sulfate (Li2SO4). (e) An aqueous solution of lead(II) acetate (Pb(C2H3O2)2) reacts with an aqueous solution of cesium bromide (CsBr). 9.76 Silver iodide (AgI) is the least soluble of all the silver halides. Silver bromide (AgBr) is slightly more soluble, and silver chloride (AgCl) is more soluble. Explain the solubility trend AgCl > AgBr > AgI. 9.77 Write net ionic equations for each of the following reactions, and tell which solubility rule or rules are applicable. (a) An aqueous solution of sodium bromide reacts with an aqueous solution of silver nitrate. (b) An aqueous solution of potassium sulfide reacts with an aqueous solution of copper(II) sulfate. (c) An aqueous solution of ammonium phosphate reacts with an aqueous solution of magnesium perchlorate. (d) An aqueous solution of barium chloride reacts with an aqueous solution of lead(II) chlorate. (e) An aqueous solution of mercury(I) acetate reacts with an aqueous solution of lithium iodide 9.8 Summary 9.78 For the representative elements, how does the number of valence electrons relate to the position of the element on the periodic table? 9.79 Give the electron configuration for each of the following ions: (a) F – (b) Mg2+ (c) P3– (d) Fe2+ (e) Ti4+ 9.80 Use arrows to indicate the direction the electrons will shift in each of the following polar bonds. (The arrows should go from the less electronegative to the more electronegative atom.) (a) O-As (b) Cl-F (c) O-C (d) S-Al (e) CH. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 75 9.81 Identify the ions that do not have a noble-gas electron configuration in each of the following compounds: (a) FeO (b) Sc2O3 (c) CuF2 (d) V2O5 (e) MnF3 9.82 What type of bonding is expected to occur in each of the following compounds: (a) Mg 3N2 (b) CuZn (c) ClF3 (a) Ionic (b) Metallic (c) Covalent 9.83 In each of the following compounds identify to which main group column on the periodic table the element X belongs: (a) CX4 (b) X3(PO4)2 (c) X2S (d) AlX (e) B2X3 (a) 7A (17) (b) 2A (2) (c) 1A (1) (d) 5A (15) (e) 6A (16) 9.84 How many protons, neutrons, and electrons are present in each of the following species? (a) 31P3– (b) 42Ca2+ (c) 14 C (d) 170Tm3+ (e) 125Te2– 9.85 Name or give the formula for each of the following compounds. (a) potassium phosphate (b) calcium nitrite (c) aluminum chloride (d) barium carbonate (e) cadmium acetate (f) KO 2 (g) Mg3N2 (h) SrS (i) (NH4)H2PO4 (j) Mg(HCO3)2 9.86 Name or give the formula for each of the following compounds. (a) ammonium oxalate (b) cesium dichromate (c) zinc cyanate (d) aluminum thiocyanate (e) ammonium bicarbonate (f) MgSO 3 (g) AlAsO4 (h) (NH4)3PO4 (i) BaC2O4 (j) BeCl2 9.87 (a) Write the electron configuration of phosphorus. (b) Indicate the valence electrons in your electron configuration of phosphorus. (c) How many electrons does phosphorus need to gain to achieve an octet? 9.88 Which of the following compounds only contain ions that obey the octet rule? (a) CsI (b) FeCl2 (c) LiH (d) ScCl3 (e) SnF2 9.89 The lattice energy of CsF2 would be greater than that of CsF. In view of this, why does CsF 2 not form? 9.90 (a) A 0.500-g sample of sodium metal was placed in a bomb calorimeter and reacted with an excess of fluorine gas. The temperature of the calorimeter rose from 23.25°C to 28.25°C. The heat capacity of the calorimeter was 3.97 kJ/°C. Determine the heat of formation of sodium fluoride. (b) Determine the lattice energy for sodium fluoride. Use the following information: ionization energy of sodium, 496 kJ/mol; electron affinity of fluorine, −328 kJ/mol; sublimation energy of sodium, 107.76 kJ/mol; bond energy of fluorine, 150.6 kJ/mol. 9.91 (a) Write balanced chemical equations for each step in the Born-Haber cycle for aluminum sulfide. Begin the process with aluminum metal, Al, and sulfur, S8. (b) Label each of your equations as to type. (For example, is the reaction a lattice energy reaction?) 9.92 The ionization energy of sodium in the Born-Haber cycle is 496 kJ/mol. (a) How much energy in joules is necessary to ionize a sodium atom? (b) What is the frequency of a photon of this energy? (c) What is the wavelength of this photon in nanometers? 9.93 (a) What volume, in milliliters, of hydrogen chloride gas at 745 mmHg and 27°C is necessary to precipitate all the lead ions from 150.0 mL of a 0.15 M lead(II) nitrate? (b) What is the percent yield if only 975 mL formed? 9.94 Write balanced chemical equations for each of the following. Give the Lewis symbols for all reactants and products. (a) the first ionization of aluminum (b) the second ionization of aluminum (c) the third ionization of aluminum 9.95 A 0.500-g sample of a compound containing aluminum and oxygen was decomposed to the elements. After the decomposition, 178.6 mL of oxygen gas at 765 torr and 25°C was isolated. Write the Lewis structure for the compound. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 76 9.96 Two 0.750-gram samples (A and B) of compounds containing lead and fluorine was decomposed to the elements. The released fluorine from compound A was sealed in a 175.0 mL metal cylinder at 30.0°C. The pressure of the fluorine released from compound A was 0.753 atm. The fluorine released from compound B was sealed in a 195.0 mL metal cylinder at 30.0°C. The pressure of the fluorine from compound B had a pressure of 0.387 atm. Draw the Lewis structures of compounds A and B. 9.98 The most stable ions of the first-row transition elements are Sc3+, Ti4+, V5+, Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Cu2+, and Zn2+. (a) Write electron configurations for each of these ions. (b) Which of the most stable ions of the first-row transition elements obeys the octet rule? 9.99 A sample of a lead compound was decomposed to its elements. The decomposition yielded lead metal and chlorine gas. The decomposition of 1.000 g of this compound yielded 143.9 mL of chlorine gas measured at 27°C and 745 mmHg. What is the formula of the compound and which of the ions in the lead compound obeyed the octet rule? 9.100 Many compounds of silver are insoluble in water. One exception to this generalization is silver fluoride (AgF). Why might silver fluoride be an exception? 9.101 Using Lewis symbols write a balanced chemical equation for the reaction of aluminum atoms with oxygen atoms to form a compound. Chapter 10 10.1 (a) What are the three different types of bonding? (b) What role does electronegativity play in these bonding types? 10.2 (a) In what type of bonding are electrons transferred? (b) In what type of bonding are electrons shared? 10.3 How is the Lewis structure of a molecule related to the Lewis structures of atoms? 10.4 In combination with Lewis structures, what two additional tools can be used to refine the understanding of molecules relative to their stability? 10.5 What theory applies quantum mechanics to molecules? 10.6 The modern explanation of metallic bonding relies very strongly upon what theory? 10.2 Corresponds to BLBMWS Sections 8.3, 8.5, and 8.7 10.7 What electronegativity difference separates ionic from covalent bonding? 10.8 If the electronegativity difference between two atoms is 0.5, how could you predict if the bonding will be covalent or metallic? 10.9 (a) Which elements, commonly considered transition metals, may be treated as representative elements when it comes to Lewis structures? (b) Write the electron configurations of these elements. 10.10 In the chlorine molecule, how many electrons are not being shared? 10.11 What is the difference between a bonding pair and a lone pair of electrons? 10.12 What is the difference between a single, a double, and a triple bond? 10.13 What is the maximum number of bonds that may be present between two representative element atoms? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 77 10.14 (a) What is the "new" definition of the octet rule given in this chapter? (b) How does the octet rule in this chapter differ from the octet rule in Chapter 9? (c) What element or elements are always an exception to the octet rule? 10.15 Write a Lewis structure for each of the following molecules or ions: (a) hydrogen sulfide (H 2S) (b) dichlorine oxide (Cl2O) (c) carbon monoxide (CO) (d) sulfate ion (SO42–) (e) nitrous acid (HNO2) 10.16 Draw a Lewis structure for each of the following molecules or ions: (a) hydrocyanic acid (HCN) (b) hydrazine (N2H4) (the nitrogen atoms are bonded to each other) (c) chlorate ion (ClO 3–) (d) ethylene (C2H4) (the carbon atoms are bonded to each other) (e) thionyl chloride (SOCl2) 10.17 Diagram the reaction of silicon atoms with fluorine atoms using Lewis symbols. 10.18 Phosphorus will combine with hydrogen to form phosphine (PH 3). Use Lewis symbols or structures to illustrate the reaction of phosphorus and hydrogen atoms to form phosphine. 10.19 Draw a Lewis structure for each of the following molecules or ions. (a) phosphorus trichloride (PCl3) (b) hydrazoic acid (HN3) (c) nitric acid (HNO3) (d) ozone (O3) (e) oxalic acid (H2C2O4) (there are no oxygen-oxygen bonds) 10.20 Even in Lewis structures that are exceptions to the octet rule, there is always one type of atom that will obtain its octet if possible. What type of atom will achieve its octet in these structures? 10.21 (a) Define a coordinate covalent bond. (b) Once formed, how does a coordinate covalent bond differ from other covalent bonds? 10.22 Which of the following elements can exceed an octet in at least some of their compounds? (a) Xe (b) C (c) N (d) S (e) B (f) Li (g) As (h) Si (i) O (j) I Can exceed Xe, S, As, Si, and I 10.23 Write a Lewis structure for each of the following and identify any atom not obeying the octet rule: (a) sulfur tetrafluoride (SF4) (b) boron trifluoride (BF3) (c) nitrogen oxide (NO) (d) chlorine dioxide (ClO2) (e) xenon tetrafluoride (XeF4) 10.24 Write a Lewis structure for each of the following and identify any atom not obeying the octet rule: (a) beryllium chloride (BeCl2) (b) krypton difluoride (KrF2) (c) bromine dioxide (BrO2) (d) tellurium tetrafluoride (TeF4) (e) aluminum fluoride (AlF3) 10.25 Write a Lewis structure for each of the following and identify which atoms, if any, which do not obey the octet rule: (a) triiodide ion (I3–) (b) beryllium hydride (BeH2) (c) germanium tetrachloride (GeCl4) (d) hexafluoroarsenate ion (AsF6–) (e) hexafluorosilicate ion (SiF62–) 10.3 Corresponds to BLBMWS Section 8.5 10.26 When determining the formal charge, how do you find each of the following? (a) The number of valence electrons an atom possesses. (b) The number of bonding electrons an atom possesses. (c) The number of nonbonding electrons an atom possesses. 10.27 Write the equation for determining the formal charge on an atom. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 78 10.28 The following sets of formal charges were found for three atoms in a triatomic ion. Tell why each set will indicate a poor Lewis structure. (a) –1, –1, +1 (b) –2, +3, –2 (c) –2, 0, +1 10.29 Draw the Lewis structure for each of the following, and predict the formal charge on each atom. (a) nitrite ion (NO2–) (b) sulfuric acid (H2SO4) (c) ozone (O3) (d) hydrogen phosphate ion (HPO42–) (e) hydrogen carbonate ion (HCO3–). 10.30 Draw Lewis structures for each of the following assuming the atomic arrangement is exactly as shown. Determine the formal charge for each atom in each structure. Based on the formal charges predict which member of each set is the more stable. (a) nitrosyl fluoride: NOF or ONF (b) dinitrogen oxide: NON or NNO (c) cyanate ion: OCN–, CNO–, or CON– (d) carbon dioxide: OCO or COO (e) cyanamide ion: CNN 2– or NCN2–. (a) NOF N = 5 – 4 – (1/2)(4) = –1 O = 6 – 2 – (1/2)(6) = +1 F = 7 – 6 – (1/2)(2) = 0 ONF More stable – lower (0) charges N = 5 – 2 – (1/2)(6) = 0 O = 6 – 4 – (1/2)(4) = 0 F = 7 – 6 – (1/2)(2) = 0 N = 5 – 4 – (1/2)(4) = –1 O = 6 – 0 – (1/2)(8) = +2 NNO More stable O not +2 Na = 5 – 0 – (1/2)(8) = +1 Nb = 5 – 2 – (1/2)(6) = 0 O = 6 – 6 – (1/2)(2) = –1 (b) NON (c) OCN– CON– More reasonable charges (no ±2) O = 6 – 4 – (1/2)(4) = 0 CNO– O = 6 – 4 – (1/2)(4) = 0 C = 4 – 0 – (1/2)(8) = 0 C = 4 – 4 – (1/2)(4) = –2 N = 5 – 4 – (1/2)(4) = –1 N = 5 – 0 – (1/2)(8) = +1 O = 6 – 0 – (1/2)(8) = +2 C = 4 – 4 – (1/2)(4) = –2 N = 5 – 4 – (1/2)(4) = –1 (d) OCO More stable – lower (0) charges COO C = 4 – 4 – (1/2)(4) = –2 C = 4 – 0 – (1/2)(8) = 0 Oa = 6 – 0 – (1/2)(8) = +2 O = 6 – 4 – (1/2)(4) = 0 Ob = 6 – 4 – (1/2)(4) = 0 (e) NCN2– More reasonable charges (no ±2) C = 4 – 0 – (1/2)(8) = 0 N = 5 – 4 – (1/2)(4) = –1 CNN2– C = 4 – 4 – (1/2)(4) = –2 Na = 5 – 0 – (1/2)(8) = +1 Nb = 5 – 4 – (1/2)(4) = –1 10.4 Corresponds to BLBMWS Section 8.6 10.31 What term describes a molecule or ion that has more than one favorable Lewis structure? 10.32 Why is it not possible for the structures HOCN and HCNO (atoms arranged as written) to be related by resonance? 10.33 What is the difference between a resonance structure and a resonance hybrid? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 79 10.34 Write a Lewis structure for each of the resonance forms for each of the following molecules or ions: (a) nitrate ion (NO3–) (b) cyanate ion (OCN–) (c) bicarbonate ion (HCO3–) (d) nitrogen dioxide (NO2) (e) dinitrogen oxide (N2O; one nitrogen is central). (a) (b) (c) (d) (e) 10.35 Use Lewis structures to arrange the following in order of decreasing carbon-oxygen bonds lengths: CO32–, CO2, CO, and CHO2– (all atoms are attached to the carbon atom). 10.5 Corresponds to BLBMWS Section 8.8 10.36 (a) Define bond energy. (b) Are bond energy values endothermic or exothermic? 10.37 Why is a heat of reaction determined from bond energies less reliable than a heat of reaction determined with a calorimeter? 10.38 What is the relationship between bond length and bond energy? 10.39 If there is resonance present, how is the bond length affected? 10.40 Using the table given in the text, calculate the bond length of each of the following: (a) H-H (b) H-O (c) O-O (d) O=O (e) N-O. 10.41 Nitrogen forms compounds with hydrogen in addition to ammonia (NH 3). One of these compounds is hydrazine (N2H4) The other nitrogen-hydrogen compounds are thermally unstable and include diazene (N2H2), triazene (HNNNH2), and tetrazene (H2NNNNH2). (a) Draw Lewis structures for nitrogen gas (N2) and each of the five nitrogen-hydrogen compounds listed. (b) Rank the five structures with nitrogen-nitrogen bonds in order of decreasing bond length. (c) Rank the five structures with nitrogen-nitrogen bonds in order of increasing bond strength. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 80 10.42 Estimate the enthalpy change for each of the following reactions based upon bond energies. (a) 2 H-H + O=O 2 H-O-H (b) 2 H-N=N-H + O=O NN + 2 H-O-H (c) H O H (a) –484 kJ C H + O O O H + H H (b) 109 kJ C H H (c) –382 kJ 10.43 Estimate the enthalpy change for each of the following reactions based upon bond energies. (a) 2 CO + O2 2 CO2 (b) 2 HCN + 5 F2 2 CF4 + N2 + 2 HF 10.44 Balance the following equations and estimate the enthalpy change for each of the following reactions based upon bond energies. (a) C2H6 + O2 CO2 + H2O (b) NH3 + F2 N2 + HF 10.6 Corresponds to BLBMWS Section 9.6 10.45 Define a bond. Sketch a bond formed from s-orbitals. 10.46 Define a bond. Sketch a bond. 10.47 Sketch how a bond could form in each of the following cases. (a) two s orbitals overlap (b) two p orbitals overlap (c) an s and a p orbital overlap 10.48 Sketch how a bond could form when two p orbitals overlap. 10.49 What type of bond restricts rotation about a bond in a molecule? Why? 10.50 If there are two bonds between two atoms, how are the two bonds oriented in relation to each other? 10.51 (a) What is the maximum number of bonds that may be present between two representative element atoms? (b) How many of these are σ bonds and how many are π bonds? 10.7 Corresponds to BLBMWS Section 16.11 10.52 (a) What is a Lewis acid? (b) What is a Lewis base? 10.53 How does the concept of coordinate covalent bonding relate to Lewis acid-base reactions? 10.54 Is it possible for a Lewis base to be a "normal" base? Explain. 10.55 Explain why a molecule with no lone pairs cannot serve as a Lewis base. 10.56 Many Lewis acids are cations. Why? 10.57 In each of the following reactions, which of the reactants is the Lewis acid and which of the reactants is the Lewis base? (a) FeCl3(s) + Cl–(aq) FeCl4–(aq) (b) H2O(l) + NO2–(aq) HNO2(aq) + OH–(aq) (c) NH3(g) + BF3(g) H3NBF3(s) (d) BrF3(l) + HF(l) H+(sol) + BrF4–(sol) (sol = solvated) (e) CaO(s) + H2O(l) Ca(OH)2(s) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 81 10.58 In each of the following reactions, which of the reactants is the Lewis acid and which of the reactants is the Lewis base? (a) SiF4(g) + 2 F–(aq) SiF62–(aq) (b) H2O(l) + NH2–(aq) OH–(aq) + NH3(aq) 2+ 2+ (c) Cu (aq) + 4 NH3(aq) Cu(NH3)4 (aq) (d) OH–(aq) + NH4+(aq) NH3(g) + H2O(l) (e) CO2(aq) + H2O(l) H2CO3(aq) 10.8 Corresponds to BLBMWS Sections 9.7-9.8 10.59 Which of the following guidelines, introduced in the chapters on atomic orbitals, apply to molecular orbitals? (a) Hund's rule; (b) the aufbau principle; (c) the Pauli exclusion principle 10.60 How do the labels of molecular orbitals differ from the labels for atomic orbitals? 10.61 When two atoms come together, molecular orbitals form. (a) Describe the similarities and differences between the atomic orbitals and the molecular orbitals. (b) Compare the bonding to the antibonding molecular orbitals. 10.62 (a) Diagram the formation of a σ bonding and a σ antibonding molecular orbital from two s orbitals. (b) Diagram the formation of a σ bonding and a σ antibonding molecular orbital from two p orbitals. 10.63 Diagram the formation of a π bonding and a π antibonding molecular orbital from two p orbitals. 10.64 (a) How do electrons in bonding molecular orbitals affect the stability of a molecule or ion? (b) How do electrons in antibonding molecular orbitals affect the stability of a molecule or ion? 10.65 What is the equation for calculating bond order? 10.66 (a) Sketch the two arrangements of the molecular orbitals arising from p atomic orbitals. (b) Beside each arrangement, list the second-period elements that use the arrangement. 10.67 What does a bond order of 0 tell you about the stability of a molecule? 10.68 The oxygen molecule is known to be paramagnetic. Which theory (Lewis or molecular orbital) best explains this property of oxygen molecules? 10.69 (a) Which diatomic molecules of the second-period elements are paramagnetic? (b) Which diatomic molecules of the second-period elements are diamagnetic? 10.70 Draw molecular orbital energy level diagrams for each of the following: (a) C 2 (b) CN– (c) NO+ (d) BF (e) N22–. Predict the bond order in each case. 10.71 Oxygen not only occurs as diatomic molecules (O2) but also in the following diatomic ions: O2+, O2–, and O22–, which are the dioxygenyl, superoxide, and peroxide ions, respectively. (a) Draw molecular orbital energy level diagrams for the oxygen molecule and each of the three ions. (b) List the four in order of increasing bond strength. (c) List the four in order of decreasing bond length. (d) Describe each of the four as either paramagnetic or diamagnetic. 10.72 Nitrogen and oxygen not only form diatomic molecules, NO, but also in the following diatomic ions: NO +, NO–, and NO2+. (a) Draw molecular orbital energy level diagrams for the NO molecule and each of the three ions. (b) List the four in order of increasing bond strength. (c) List the four in order of decreasing bond length. (d) Tell which of the four is paramagnetic and which is diamagnetic. 10.73 What is the maximum number of electrons that may be accommodated by each of the following: (a) a bonding molecular orbital (b) a -antibonding molecular orbital? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 82 10.9 Corresponds to BLBMWS Section 12.4 10.74 How does the molecular orbital energy-level diagram for metallic bonding differ from that of a simple diatomic molecule? 10.75 What is a conduction band? 10.76 Beginning with a single lithium (Li) atom, show how an energy band forms when more lithium atoms come together. 10.10 Summary 10.77 Define valence band and band gap. 10.78 Sketch the band structure for germanium. 10.79 (a) Sketch the band structure for silicon. (b) Make another sketch showing how the band structure of silicon containing a small quantity of phosphorus differs from that of pure silicon. (c) Make another sketch showing how the band structure of silicon containing a small quantity of aluminum differs from that of pure silicon. 10.80 (a) How does the band structure of a p-type semiconductor differ from a normal (undoped) semiconductor? (b) How does the band structure of an n-type semiconductor differ from a normal semiconductor? 10.81 Describe how p-type and n-type semiconductors differ from a pure metalloid such as silicon. 10.82 Determine which of the following are n-type and which are p-type semiconductors: (a) germanium doped with arsenic (b) silicon doped with aluminum (c) germanium doped with gallium (d) silicon doped with phosphorus (e) diamond doped with nitrogen. 10.83 The density of ozone gas is 2.144 g/L. What is the density of ozone in pounds per cubic foot? 10.84 The blue color of ozone is produced by the absorption of red light in the 557- to 602-nm region. (a) What is the frequency of a 557-nm wave of red light? (b) What is the energy, in joules, of a red photon with a wavelength of 602 nm? 10.85 Ozone absorbs ultraviolet radiation in the 200- to 310-nm region. (a) What is the frequency of a 275-nm wave of ultraviolet light? (b) What is the energy, in joules, of an ultraviolet photon with a wavelength of 285 nm? (c) How many kilojoules will your skin receive if it absorbs 1.00 × 10 –3 mol of photons from 200.0-nm ultraviolet light? 10.86 The standard enthalpy of formation for ozone is 143 kJ/mol O 3. (a) Use bond energies to estimate the standard enthalpy of formation for ozone. (b) The difference between the standard enthalpy of formation of ozone and the estimated value from part (a) is primarily due to resonance. Calculate how much resonance contributes to the stability of ozone. 10.87 It is possible to produce ozone by the reaction of oxygen gas with oxygen atoms. O2(g) + O(g) O3(g) H = 105 kJ/mol O3 (a) Use bond energies to estimate the standard enthalpy for this reaction. (b) The difference between the standard enthalpy of formation of ozone and the estimated value from part (a) is primarily due to resonance. Calculate the extent to which resonance contributes to the stability of ozone. (a) –142 kJ (b) –247 kJ 10.88 For ozone to be produced through the reaction of oxygen atoms with oxygen molecules in the upper atmosphere, oxygen atoms must first be produced from oxygen molecules. The bond energy in an oxygen molecule is 498.7 kJ/mol. What is the maximum wavelength, in nanometers, of a photon with sufficient energy to break the bond in an oxygen molecule? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 83 10.89 Ozone is a powerful oxidizing agent. Balance the following reactions of ozone. (a) NO2(g) + O3(g) N2O5(s) + O2(g) (b) KOH(aq) + O3(g) KO3(s) + O2(g) + H2O(g) (c) Ag(s) + O3(g) Ag2O(s) + O2(g) (d) KOCN(aq) + H2O(l) + O3(g) KHCO3(aq) + O2(g) + N2(g) (e) Hg(l) + O3(g) HgO(s) 10.90 The compound potassium ozonide (KO3) forms when ozone reacts with potassium metal. (a) Draw a Lewis structure for the ozonide ion. (b) The ion is known to be paramagnetic. Does your Lewis structure support this observation? 10.11 Summary 10.91 Rank the following bonds in order of increasing polarity: N-P, N-N, N-O, N-F. 10.92 Show how Lewis symbols combine to construct the products (Lewis structures) of each of the following reactions. Begin with individual atoms of the elements involved and end with the appropriate compound. (a) Chlorine reacts with fluorine to produce ClF3. (b) Aluminum reacts with fluorine to produce AlF3. 10.93 Use Lewis structures to indicate the reaction in each of the following. Begin with individual atoms of the elements involved and end with the appropriate compound. (a) Bromine reacts with fluorine to produce bromine pentafluoride. (b) Aluminum reacts with oxygen to produce aluminum oxide. (a) (b) 10.94 Draw Lewis structures for each of the following. (a) carbon monoxide (CO) (b) methane (CH 4) (c) dimethylberyllium (Be(CH3)2) (d) xenon tetraoxide (XeO4) (e) paraperiodic acid (H5IO6) 10.95 For many years, it was thought that the simplest boron hydride was BH 3. After much work, it was found that the simplest boron hydride is diborane (B 2H6). (a) Draw a Lewis structure for BH3. (b) What problem occurs when you attempt to draw a Lewis structure for diborane? 10.96 A gas with a formula weight of about 166 g/mol is found to be 14.49 % carbon and 85.51 % chlorine. Draw its Lewis structure of the molecule. 10.97 Oxalic acid (H2C2O4) is a diprotic acid. It may lose one H+ to form the hydrogen oxalate ion (HC2O4–) or two H+ to form the oxalate ion (C2O42–). In the acid, the two carbon atoms are bonded to each other, and each carbon has one O-H group attached. (a) Draw the Lewis structures, showing all resonance forms, for H 2C2O4, HC2O4–, and C2O42–. (b) Compare the relative C-O bond lengths in each of the three substances. 10.98 The Haber process is used industrially to synthesize ammonia from the elements. The reaction is N2(g) + 3 H2(g) 2 NH3(g) (a) Calculate the heat of reaction using H°f values from Appendix C. (b) Calculate the heat of reaction using bond energies. (c) Account for any discrepancies between your answers for (a) and (b). 10.99 Draw the Lewis structures for each of the following compounds: (a) sodium chloride (NaCl) (b) magnesium bromide (MgBr2) (c) sodium nitrate (NaNO3) (d) potassium sulfate (K2SO4) (e) ammonium phosphate ((NH4)3PO4) 10.100 Draw Lewis structure for the following compounds: (a) potassium fluoride (b) magnesium nitride (c) sodium carbonate (d) ammonium sulfate (e) calcium phosphate © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 84 10.101 Name or give the formula for each of the following compounds. (a) potassium peroxide (b) calcium phosphate (c) aluminum sulfide (d) barium chloride (e) cadmium arsenate (f) PbO 2 (g) Mg(NO2)2 (h) SrSO4 (i) (NH4)2HPO4 (j) Mg(HSO3)2 10.102 Name or give the formula for each of the following compounds. (a) ammonium carbonate (b) cesium chromate (c) zinc thiocyanate (d) aluminum chlorate (e) ammonium bromate (f) MgSO 4 (g) AlPO4 (h) (NH4)H2AsO4 (i) BaCr2O7 (j) Be(ClO2)2 10.103 What is the name or the formula of each of the following compounds: (a) CrCl 3 (b) ZnCl2 (c) Mn(ClO3)2 (d) CoSO3 (e) AgF2 (f) manganese(VII) oxide (g) lead(IV) fluoride (h) iron(III) perchlorate (i) silver(II) oxide (j) nickel(II) thiocyanate 10.104 What is the name or the formula of each of the following compounds: (a) molybdenum(VI) oxide (b) V(ClO4)3 (c) Cu2S (d) iron(II) chlorite (e) Pb(BrO) 2 (f) titanium(III) thiosulfate (g) CrO3 (h) iron(III) arsenate (i) tungsten(IV) oxide (j) MnO2 10.105 The amount of ozone in air may be determined by bubbling the air through a basic potassium iodide solution. The reaction is O3(g) + 2 KI(aq) + H2O(l) I2(aq) + 2 KOH(aq) + O2(g) The liberated iodine is measured by a titration with a sodium thiosulfate solution. The reaction is I2(aq) + 2 Na2S2O3(aq) 2 NaI(aq) + Na2S4O6(aq) (a) Titration of the released iodine required 45.25 mL of 0.01778 M sodium thiosulfate solution. How many moles of iodine had been released by the ozone? (b) The iodine was produced by the passage of 1.00 × 10 5 L of air at 748 mmHg and 27°C through the potassium iodide solution. What is the volume of the ozone present at the same pressure and temperature as the air? (c) Determine the concentration of the ozone in the air sample in parts per million. Hint: Divide the volume of ozone by the volume of the air, and multiply the result by one million. (d) Would this level of ozone be easily detectable by a person? 10.106 A sample of a xenon fluoride partially reacts with water to produce a compound containing xenon, oxygen, and fluorine. Analysis of the compound showed it to have a molar mass of 220 g/mole and to contain 58.8% Xe, 7.2% O, and 34.0% F, by mass. Draw the Lewis structure of compound. 10.107 Draw the Lewis structures for three different compounds having a molar mass of about 60 g/mole and containing 59.96% C, 13.42% H, and 26.63% O, by mass. 10.108 In which of the following does the central atom obey the octet rule? (a) Methane (b) Boron trifluoride (c) Sulfur tetrafluoride (d) Ammonia (e) Arsenic pentafluoride 10.109 Arsenic, for semiconductors, occurs naturally as the mineral realgar, AsS. If realgar is heated in air, atmospheric oxygen reacts to produce sulfur dioxide gas and solid arsenic(III) oxide. The arsenic(III) oxide is mixed with carbon and heated to produce solid elemental arsenic and gaseous carbon monoxide. Write balanced chemical equations for these reactions. Chapter 11 11.1 Corresponds to BLBMWS Section 9.1 11.1 What is the definition of molecular geometry? 11.2 What key information does the VSEPR approach rely upon? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 85 11.3 What key information does the valence bond approach rely upon? 11.4 What behavior characterizes the interaction of polar molecules? 11.2 Corresponds to BLBMWS Section 9.1 11.5 When does a polar covalent bond form? 11.6 Consider the molecules H2 and ClF. (a) Which has a polar covalent bond? (b) Which is a polar molecule? 11.7 (a) Sketch how an H2S molecule might appear if it were nonpolar. (b) Sketch how an H 2S molecule might appear if it were polar. 11.8 The molecule IF is polar. Show two methods of illustrating that it is polar. 11.9 What are the definitions of dipole and dipole moment? 11.10 What effect does the polarity of water have upon the boiling point of water? 11.11 Place the members of each of the following sets in order of decreasing polarity of the bond shown. (a) I-O, ClO, and Br-O (b) C-N, C-C, and C-O (c) C-H, O-H, and F-H (d) F-F, B-F, and Li-F (e) Na-H, N-N, and C-S (a) Cl-O > Br-O > I-O (b) C-O > C-N > C-C (c) F-H > O-H > C-H (d) Li-F > B-F > F-F (e) Na-H > C-S > N-N 11.3 Corresponds to BLBMWS Section 9.2 11.12 (a) What does the acronym VSEPR stand for? (b) To what does the VS in VSEPR refer? (c) To what does the EP in VSEPR refer? (c) To what does the R in VSEPR refer? 11.13 What two terms may be used to describe the positions of electron groups about the central atom? 11.14 (a) The greatest repulsion occurs between which types of electron pairs? (b) The smallest repulsion occurs between which types of electron pairs? (c) Which electron pair combination yields an intermediate level of repulsion? 11.15 Which type of repulsion does the VSEPR model try hardest to reduce? 11.4 Corresponds to BLBMWS Section 9.5 11.16 What is the definition of hybridization? 11.17 If an atom hybridizes five atomic orbitals, how many hybrid orbitals will form? 11.18 What things change when atomic orbitals hybridize? 11.5 Corresponds to BLBMWS Sections 9.2-9.6 11.19 (a) What orbital geometry results when the central atom has two electron pairs? (b) What molecular geometry results when the central atom has two electron pairs and no lone pairs? 11.20 What bond angle results when the central atom has two electron pairs? 11.21 What hybridization about a central atom surrounded by two electron pairs? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 86 11.22 The compound cadmium iodide (CdI2) may be treated as covalent. (a) Write the electron configuration for cadmium. (b) Draw a Lewis structure for cadmium iodide. (c) What is the orbital geometry about the cadmium in the compound? (d) What is the molecular geometry of cadmium iodide? (e) What is the hybridization of cadmium in cadmium iodide? (f) Is cadmium iodide polar or nonpolar? (a) 1s22s22p63s23p64s23d104p65s24d10 or [Kr]4s24d10 (c) Linear (d) sp (e) Nonpolar (b) 11.6 Corresponds to BLBMWS Sections 9.2-9.6 11.23 (a) What is the orbital geometry resulting when the central atom has three electron pairs? (b) What is the molecular geometry when the central atom has three electron pairs and no lone pairs? (c) What is the molecular geometry when the central atom has three electron pairs and one lone pair? 11.24 (a) What is the bond angle when a central atom has three electron pairs? (b) How does the bond angle change if one of the pairs is a lone pair? 11.25 What is the hybridization about a central atom surrounded by three electron pairs? 11.26 Predict the orbital geometry and the molecular geometry of each of the following. (a) boron trifluoride (BF 3) (b) nitrate ion (NO3–) (c) nitrosyl chloride (NOCl) (d) hydrocyanic acid (HCN) (e) formate ion (CHO 2–) 11.27 The compound B2Cl4 is unusual in that the boron atoms are bonded to each other. Identify the orbital and molecular geometry around each boron atom. 11.28 Predict the hybridization about the central atom in each of the following. (a) boron trifluoride (BF3) (b) nitrate ion (NO3–) (c) nitrosyl chloride (NOCl) (d) hydrocyanic acid (HCN) (e) formate ion (CHO 2–) 11.7 Corresponds to BLBMWS Sections 9.2-9.6 11.29 (a) What orbital geometry is produced when the central atom has four electron pairs? (b) What is the molecular geometry when the central atom has four electron pairs and no lone pairs? (c) What is the molecular geometry when the central atom has four electron pairs and one lone pair? (d) What is the molecular geometry when the central atom has four electron pairs and two lone pairs? 11.30 (a) What bond angle occurs in a molecule when the central atom has four electron pairs? (b) How does the bond angle change if one of the pairs is a lone pair? (c) How does the bond angle change if two of the pairs are lone pairs? 11.31 What is the hybridization about the central atom if there are four electron pairs about it? 11.32 Carbon is the only element that readily forms bonds with itself. The ability of an unlimited number of carbon atoms to bond together is very important to organic chemistry and biochemistry. Predict the orbital geometry, molecular geometry, and hybridization about the carbon atoms in each of the following compounds, all of which contain carbon atoms bonded to each other. (a) ethane (C2H6) (b) ethene (C2H4) (c) ethyne (C2H2) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 87 11.33 Acetic acid (HC2H3O2) is an asymmetrical compound in which the two carbons are connected. One carbon has three of the hydrogen atoms attached, while the other carbon has the two oxygen atoms attached to it. One of the oxygen atoms has the fourth hydrogen (the acid hydrogen). (a) Draw the Lewis structure of acetic acid. (b) What is the orbital geometry about each carbon atom? (c) What is the molecular geometry about each carbon atom? (d) What is the hybridization about each carbon atom? 11.34 A measure of the polarity of a molecule is its dipole moment. The greater the dipole moment, the more polar the molecule is. The unit used to express the dipole moment is the debye (abbreviated D). Carbon tetrachloride (CCl4) has no dipole moment. However, when one of the chlorines is replaced with a hydrogen atom, the new molecule has a dipole moment of 1.01 D. Explain the change in polarity. 11.35 Dichloroethane, with the general formula C2H2Cl2, has three isomers (different compounds with the same formula). Draw the structures of the three isomers of dichloroethene and predict which are polar. 11.36 Draw Lewis structures for each of the following and indicate the approximate bond angles throughout the molecule: (a) nitric acid (HNO3) (b) hydroxylamine (NH2OH) (c) ethanol (CH3CH2OH) (d) acetylene (HCCH) (e) ethyl acetate (CH3CH2OC(=O)CH3). In each case, the hydrogens are next to the atom they bond. The "=O" stands for an oxygen double bonded to the carbon preceding it. 11.37 Why are the electron pair and molecular geometries not always the same? Use methane (CH 4) and water (H2O) in your explanation. 11.38 The bond angle in methane is the ideal tetrahedral angle of 109.5°. Why are bond angles in other compounds with tetrahedral orbital geometries not always ideal? 11.39 Carbon often occurs with sp3 hybridization; however, it may also adopt sp and sp2 hybridization. (a) How many unhybridized p orbitals are in the valence shell of carbon in each of the three hybridization schemes (sp3, sp2, and sp)? (b) How many bonds can a carbon atom form in each of these hybridization schemes? (c) What is the maximum number of bonds a single carbon atom can form? (a) sp3 = 0, sp2 = 1, and sp = 2 (b) sp3 = 0, sp2 = 1, and sp = 2 (c) 2 11.40 The structure of ethyl acetate is Each carbon and the oxygen bonded to two different carbons may be treated as a central atom. (a) Give the hybridization of each central atom. (b) Give the approximate bond angles about each central atom. (c) What is the total number of bonds present? (d) What is the total number of bonds present? 11.41 The amino acid tryptophan has the structure shown below. What is the orbital and molecular geometry around each number atom? 11.42 Cocaine has the structure shown below. What is the hybridization of each numbered atom? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 88 11.43 The controversial drug mifepristone (RU486) has the structure shown below: (a) What is the orbital geometry around each numbered atom? (b) What is the molecular geometry around each numbered atom? (c) What is the hybridization around each numbered atom? (d) How many bonds are present? (e) How many bonds are present? 11.44 The structure of diazepam (Valium®) is: (a) What is the orbital geometry around each numbered atom? (b) What is the molecular geometry around each numbered atom? (c) What is the hybridization around each numbered atom? (d) How many bonds are present? (e) How many bonds are present? 11.8 Corresponds to BLBMWS Sections 9.2-9.6 11.45 (a) What is the bond angle(s) for a molecule when the central atom has five electron pairs? (b) How does the bond angle change if one of the pairs is a lone pair? (c) How does the bond angle change if two of the pairs are lone pairs? (d) How does the bond angle change if three of the pairs are lone pairs? 11.46 (a) What is the orbital geometry for a molecule where the central atom has five electron pairs and no lone pairs? (b) Would the orbital geometry be the same if one or more of the five pairs were a lone pair? (c) What is the molecular geometry for a molecule where the central atom has five electron pairs and no lone pairs? (d) Would the molecular geometry be the same if one or more of the five pairs were a lone pair? 11.47 What is the hybridization about a central atom surrounded by five electron pairs? 11.48 Predict the hybridization of the central atom, the molecular geometry, and the approximate bond angle for each of the following: (a) chloric acid (HClO3) (b) ammonium ion (NH4+) (c) arsenic pentafluoride (AsF5) (d) germanium tetrachloride (GeCl4) (e) ozone (O3) (f) carbonate ion (CO32–) (g) triiodide ion (I3–) (h) tetrahydridoaluminate ion (AlH4–) (i) orthonitrate ion (NO43–) (j) sulfurous acid (H2SO3) 11.49 Determine which of the following are polar: (a) hydrogen chloride (HCl) (b) nitrogen dioxide (NO 2) (c) krypton difluoride (KrF2) (d) ammonia (NH3) (e) boron trifluoride (BF3) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 89 11.50 Determine which of the following have a non-zero dipole moment: (a) carbon dioxide (CO2) (b) hypochlorous acid (HOCl) (c) iodine trichloride (ICl3) (d) trichloromethane (chloroform) (CHCl3) (e) beryllium iodide (BeI2) 11.51 When molecules of boron trifluoride (BF3) and chlorine trifluoride (ClF3) are compared, it is found that even though the electronegativity difference in the bonds is greater in BF 3, that compound is not polar, whereas ClF3 is polar. Explain. 11.9 Corresponds to BLBMWS Sections 9.2-9.6 11.52 (a) What orbital geometry results when a central atom has six electron pairs? (b) What is the molecular geometry when the central atom has six electron pairs and no lone pairs? (c) What is the molecular geometry when the central atom has six electron pairs and one is a lone pair? (d) What is the molecular geometry when the central atom has six electron pairs and two are lone pairs? 11.53 (a) What is the bond angle for a molecule when the central atom has six electron pairs? (b) How does the bond angle change if one of the pairs is a lone pair? (c) How does the bond angle change if two of the pairs are lone pairs? 11.54 What is the hybridization about the central atom if there are six electrons pairs about it? 11.55 Predict the hybridization of the central atom in each of the following: (a) carbon dioxide (CO 2) (b) bromine pentafluoride (BrF5) (c) formaldehyde (CH2O) (d) nitrate ion (NO3–) (e) tetrachloroiodate ion (ICl4–) (f) dichloromethane (CH2Cl2) (g) silane (SiH4) (h) selenium tetrafluoride (SeF4) (i) hydrocyanic acid (HCN) (j) xenon tetrafluoride (XeF4) 11.56 Draw the Lewis structure and list the electron-pair geometry and molecular geometry for each of the following: (a) hydrogen selenide (H2Se) (b) phosphine (PH3) (c) selenium tetrafluoride (SeF4) (d) triiodide ion (I3–) (e) sulfur hexafluoride (SF6) (f) thionyl chloride (SOCl 2) (g) nitrate ion (NO3–) (h) chlorine trifluoride (ClF3) (i) sulfuric acid (H2SO4) 11.57 The following species have the general molecular formulas XF 4: carbon tetrafluoride (CF4) sulfur tetrafluoride (SF4) and xenon tetrafluoride (XeF4). Determine the molecular geometry for each, and explain why they have the same general formula but differing geometries. 11.58 Determine the molecular geometry for (a) thiocyanate ion (SCN –) (b) hydrogen carbonate ion (HCO3–) (c) xenon difluoride (XeF2) (d) sulfite ion (SO32–) (e) antimony pentafluoride (SbF5) (a) (b) (c) (d) (e) (a) linear (b) trigonal planar (c) linear (d) trigonal pyramid (e) trigonal bipyramid © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 90 11.10 Summary 11.59 (a) A solution was prepared by dissolving olive green platinum(II) chloride, PtCl 2, in water. The solution was divided into two portions. Adding an excess of potassium chloride to one portion of the solution precipitated a red solid. Adding an excess of ammonia to the second portion followed by the addition of potassium bromide yielded a colorless precipitate. The analyses of these two precipitates are in the table below. Determine the empirical formulas of each of the precipitates. (b) A portion of the red solid was dissolved in a hot ammonia solution and an orange-yellow polar solid was isolated on cooling. A portion of the colorless solid was dissolved in warm hydrochloric acid and a pale-yellow nonpolar solid was isolated. Both solids had the same composition (see the table below). The molar mass of each solid was about 300 g/mole. Determine the molecular formula for each solid. (c) Assuming all the hydrogen atoms remain attached to the nitrogen as they were attached in ammonia, sketch the structures of the two solids in part (b) assuming the molecules are square planar with the platinum in the center. Analysis of the samples Red Colorless Orange-yellow Pale-yellow Solid Solid Solid Solid K 0.458 g 0 0 0 Pt 1.143 g 1.499 g 65.02 % 65.02 % Cl 0.831 g 0 23.63 % 23.63% N 0 0.216 g 9.337 % 9.337% H 0 0.094 g 2.016 % 2.016 % Br 0 1.228 g 0 0 11.11 Summary 11.60 (a) Draw Lewis structures for carbon monoxide (CO) and hydrogen chloride (HCl). (b) What is the molecular geometry of each of these molecules? (c) Why is it unnecessary to have a line in the geometry table to accommodate these electron pair combinations? 11.61 Sketch the arrangement arising when an atom has the following numbers of electron pairs about it: (a) 2 (b) 3 (c) 4 (d) 5 (e) 6. 11.62 How many electron pairs must surround the central atom to yield the following angles between the pairs: (a) 180°; (b) 120°; (c) 109.5°; (d) 90°. Some of these angles may have more than one answer. 11.63 (a) Draw a Lewis structure for the carbonate ion (CO32–). (b) The three oxygen atoms are known to be equivalent. Does your Lewis structure show equivalent oxygen atoms? (c) Beginning with the Lewis structure you drew in part (a), what needs to be done to show that the oxygen atoms are equivalent? (d) What is the hybridization on the carbon? (e) Which orbitals in your structure in part (a) are used for bonding electrons and which are used for nonbonding electrons? (f) The delocalized system utilizes which orbitals? (g) How many electrons are delocalized? 11.64 Draw the Lewis structures for the carbonate ion (CO 32–) and carbonic acid (H2CO3). Is the bond delocalized in either case? What evidence is there that the bond is delocalized? 11.65 (a) Draw resonance structures for the oxalate ion (C2O42–). The two carbon atoms are connected. (b) What is the molecular geometry about each carbon atom? (c) Determine the hybridization of each carbon atom. (d) Is the bond delocalized? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 91 11.66 In which of the following does atom A obey the octet rule? 11.67 Determine the orbital geometry around the central atom in each of the following. 11.68 Determine the hybridization around the central atom in each of the following. 11.69 (a) What is the ideal bond angle about the central atom in each of the following? (b) Are the actual bond angles expected to be ideal or not? (c) If the angles are not ideal, are they larger or smaller than expected? 11.70 Give the hybridization and the number of surrounding electron pairs for the central atom in each of the following orbital geometries: (a) octahedral; (b) trigonal bipyramidal; (c) tetrahedral; (d) trigonal planar; (e) linear. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 92 11.71 Xenon reacts with fluorine to produce various xenon fluorides. The product depends on the amount of fluorine present. The following reactions are known: Xe(g) + F2(g) XeF2(g) Xe(g) + 2 F2(g) XeF4(g) Xe(g) + 3 F2(g) XeF6(g) (a) Draw the Lewis structure for each of the three xenon fluorides. (b) Predict the molecular geometry of each of the xenon fluorides. What problem, if any, do you have with xenon hexafluoride? (c) Predict the hybridization of xenon in each of the three xenon fluorides. (d) Draw a Lewis structure for iodine heptafluoride (IF 7) and compare it to the Lewis structure of xenon hexafluoride. (e) The observed geometry for iodine heptafluoride is a pentagonal bipyramid. A pentagonal bipyramid is similar to a trigonal bipyramid except that there are five equatorial atoms instead of three. Predict the structure of xenon hexafluoride as related to that of iodine heptafluoride. 11.72 (a) Draw a Lewis structure for the nitrite ion (NO2–). (b) The two oxygen atoms are known to be equivalent. Does your Lewis structure show equivalent oxygen atoms? (c) Beginning with the Lewis structure you drew in part (a), what needs to be done to show that the oxygen atoms are equivalent? (d) What is the hybridization on the nitrogen? (e) Which orbitals in your structure in part (a) does the nitrogen use for bonding electrons and which are used for nonbonding electrons? (f) The delocalized system utilizes which orbitals? (g) How many electrons are delocalized? 11.73 What is the name or the formula of each of the following compounds: (a) MnCl 2 (b) MnCl3 (c) FeCl3 (d) CrSO4 (e) CuCl2 (f) lead(II) oxide (g) tin(IV) fluoride (h) chromium(III) oxide (i) gold(I) chloride (j) nickel(II) sulfite 11.74 What is the name or the formula of each of the following compounds: (a) manganese(III) oxide (b) SnCl 2 (c) CuOCN (d) cobalt(III) nitrate (e) PbF2 (f) copper(II) sulfide (g) MnO (h) chromium(III) sulfate (i) vanadium(IV) oxide (j) Fe2O3 11.75 What is the name or the formula of each of the following compounds: (a) Fe(ClO 2)2 (b) (NH4)2Cr2O7 (c) Fe(ClO3)3 (d) MnSO3 (e) AgF2 (f) molybdenum(IV) oxide (g) platinum(II) fluoride (h) titanium(III) oxide (i) vanadium(III) chloride (j) cadmium sulfide 11.76 What is the name or the formula of each of the following compounds: (a) chromium(III) dichromate (b) PbCl 4 (c) CuSCN (d) manganese(III) nitrate (e) PbO 2 (f) nickel(II) thiosulfate (g) PtO2 (h) chromium(III) dihydrogen phosphate (i) vanadium(IV) oxide (j) Au2O3 11.77 Boron trichloride is a Lewis acid that will react with ammonia and other Lewis bases. (a) Write a balanced chemical equation for the reaction of gaseous boron trichloride with ammonia gas to produce solid BCl 3NH3. (b) Draw Lewis structures for all reactants and products in this reaction. (c) Which atom(s) change molecular geometry during the reaction? 11.78 The compound benzene (C6H6) is an example of an organic hydrocarbon. The six carbon atoms in benzene are attached to each other at the corners of a hexagon. One hydrogen atom is bound to each of the carbon atoms. Benzene is nonpolar. If a chlorine atom replaces one of the hydrogen atoms, chlorobenzene (C 6H5Cl) results. Chlorobenzene is polar. Replacing two hydrogen atoms with chlorine atoms produces dichlorobenzene (C6H4Cl2). There are different forms of dichlorobenzene – some polar and some nonpolar. (a) Using lines for bonds draw resonance structures for benzene. (b) Using lines for bonds draw a structure for chlorobenzene. (c) Using lines for bonds draw structures for the different dichlorobenzenes. (d) Which of your structures in part (c) are polar and which are nonpolar? 11.79 The liquid compound benzene (C6H6) will burn in oxygen gas to form carbon dioxide gas and water vapor. (a) Write a balanced chemical equation for this reaction. (b) What are the molecular geometries of the products? (c) Using standard heats of formation, calculate the heat of reaction. (d) Calculate the heat of reaction using bond energies. (e) What might be the major source of the discrepancy between your answers in parts (c) and (d)? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 93 11.80 The analysis of a compound found 26.95 percent sulfur, 13.45 percent oxygen, and 59.60 percent chlorine. The molar mass is about 120 g/mol. Determine the molecular geometry around the sulfur atom The molecular geometry about the sulfur is trigonal pyramidal. 11.81 The analysis of a compound containing carbon, hydrogen, and oxygen found 52.1% C, 13.1% H, and 34.7% O. The molar mass of the compound is about 45 g/mol. There are two compounds with this molecular formula. Draw the Lewis structures for each of these two compounds. 11.82 A 2.00-mg sample of an unknown hydrocarbon was burned in oxygen. After combustion was complete, the products were analyzed and found to consist of 6.275 mg of carbon dioxide (CO 2) and 2.569 mg of water (H2O). Determine the empirical formula of the unknown hydrocarbon. The molar mass of the compound is 28 g/mol. (a) What is the molecular formula of the compound? (b) Write a balanced chemical equation for the combustion reaction. (c) Draw the Lewis structure for the compound. (d) What is the hybridization of each carbon atom? 11.83 (a) Using N2H4(l) + O2(g) N2(g) + 2 H2O(l) H = –622.2 kJ 2 H2(g) + O2(g) 2 H2O(l) H = –571.6 kJ Determine H for the following reaction: N2(g) + 2 H2(g) N2H4(l) (b) Using bond energies determine the value of H for the following reaction. N2(g) + 2 H2(g) N2H4(g) (c) Determine the hybridization of oxygen in H2O and the nitrogen in N2H4. 11.84 (a) A gas sample with a density of 1.27 g/L at 25°C and 0.955 atm was analyzed and found to be 37.4 % carbon, 12.6 % hydrogen, and 49.9 % oxygen. (a) Assuming there was only one compound in the gas sample, what is its molecular formula? (b) Determine the molecular geometry around each non-hydrogen atom. 11.85 Draw Lewis structures for each of the following assuming, the atomic arrangement is exactly as shown. Determine the formal charge for each atom in each structure. Based on the formal charges, predict which member of each set is the more stable. What is the hybridization on the central atom in each structure? (a) dinitrogen oxide: NON or NNO (b) cyanate ion: OCN–, CNO–, or CON– 11.86 (a) Draw resonance structures for the thiocyanate ion. (b) What is the hybridization of the central atom in each of the resonance structures? 11.87 Hydrochloric acid will react with sodium carbonate to produce unstable carbonic acid and sodium chloride. All species are in aqueous solution. (a) Write a balanced chemical equation for the reaction. (b) Write a net ionic equation for the reaction. (c) Identify the spectator ions in the reaction. (d) Give Lewis structures for all molecules and ions in the total ionic equation. (e) Which molecules or ions are polar? (f) How many protons, neutrons, and electrons does each of the monatomic ions possess? Assume the isotopes present in the reaction are 1H, 35Cl, 23Na, 12 C, and 16O. 11.88 Determine the hybridization about each carbon atom in each of the following. (a) methane (b) sodium bicarbonate (c) potassium cyanide (d) calcium oxalate (e) acetic acid © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 94 11.89 The NO2– and NO2+ have structures related to nitrogen dioxide (NO2). In all of them, the nitrogen is the central atom. The bond angles are 115°, 134°, and 180°. Draw Lewis structures for the three species, and match the observed bond angles with the Lewis structures. Explain any deviation from the ideal bond angle. 115° 180° 134° For NO2+ VSEPR predicts it to be linear due to the presence of the two pairs with no lone pairs For the other two, VSEPR predicts 120°; however, the lone pair in NO2– repels more strongly than the bonding pairs so the angle is slightly less than ideal. The third “pair” in NO2 is only one electron; therefore, the repulsion is less than for a pair, which leads to an angle slightly greater than ideal. 11.90 The amide ion (NH2–) and the ammonium ion (NH4+) are similar to ammonia (NH3). Draw Lewis structures for all three and match each Lewis structure with one of the following observed bond angles – 109°, 107°, and 105°. 11.91 There are a few compounds known to contain the orthonitrate ion (NO 43–). Draw a Lewis structure for this ion and predict the molecular geometry. 11.92 How many unhybridized p orbitals are present in the central atom in each of the following? (a) carbon dioxide, (b) hydrocyanic acid (c) methane (d) boron trifluoride (e) nitrogen dioxide 11.93 How many bonds the central atom form in each of the following hybridizations? (a) sp (b) sp 2 (c) sp3 (d) sp3d (e) sp3d2 11.94 Why does the VSEPR model count single, double, and triple bonds the same? 11.95 The molecule N2F2 exists in two forms. In each form, the nitrogen atoms are connected, and each nitrogen atom has a fluorine atom attached. One of the forms is polar, and the other is nonpolar. Draw the structures for the two forms and label each as polar or nonpolar. 11.96 Phosphorus forms the following pentahalides – PF5, PCl5, PBr5, and PI5. Phosphorus pentafluoride has the same structure in both the gaseous and the solid state. The other three pentahalides have the same structure as the pentafluoride in the gaseous state, but they form ionic solids. (a) Draw the Lewis structure of each of the pentahalides in the gaseous state. (b) What is the molecular geometry of each of the pentahalides in part (a)? (c) In the solid state, phosphorus pentachloride exists as PCl4+ ions and PCl6– ions. Draw the structures of these two ions and predict the molecular geometry of each. (d) The remaining pentahalides exist as ionic solids containing the PX4+ ion and the X– ion (where X = Br or I). Draw the structures of these ions and predict the structures. 11.97 Iodine trichloride (ICl3) is capable of behaving as a Lewis acid or a Lewis base. This molecule will even react with itself to form a dimer (a molecule consisting of two of the original molecules). When the dimer forms, the chlorine atom on each ICl3 behaves as a Lewis base and donates a pair of electrons to the iodine atom in the other ICl3. (a) Draw a Lewis structure of iodine trichloride. (b) Draw a Lewis structure of the dimer of iodine trichloride. (c) Predict the Lewis structure about the iodine in iodine trichloride. (d) Predict the Lewis structure about the iodine atoms in the dimer. 11.98 Cyclopropane (C3H6) is an unstable hydrocarbon with the three carbon atoms forming an equilateral triangle. Each carbon atom is attached to two hydrogen atoms. (a) Draw a Lewis structure for cyclopropane. (b) Predict the bond angle between the carbon atoms. (c) Why does your prediction in part (b) help explain why cyclopropane is unstable? 11.99 There are three compounds with the formula PCl3F2. One is nonpolar and the other two are polar. (a) Draw structures for all three compounds with the formula PCl3F2. (b) Label each of your structures in part (a) as polar or nonpolar. 11.100 There are three organic compounds with the formula C3H4. (a) Draw structures for each of these compounds. (b) What is the hybridization of each carbon atom in each of your answers to part (a)? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 95 11.101 Draw the Lewis structure for ammonium nitrate and predict the molecular geometry about each nitrogen atom. NH4+ is tetrahedral and NO3– is trigonal planar Chapter 12 12.1 Corresponds to BLBMWS Sections 11.4 and 11.6 12.1 What are the three states of matter? 12.2 What is the name associated with each of the following phase changes? (a) solid to liquid (b) liquid to gas (c) solid to gas (d) liquid to solid (e) gas to liquid (f) gas to solid (a) solid to liquid = Melting (fusion) (b) liquid to gas = Boiling (vaporization or evaporation) (c) solid to gas = Sublimation (d) liquid to solid = Freezing (crystallization) (e) gas to liquid = Condensation (f) gas to solid = Deposition 12.3 What are the two opposing factors that are important in determining the physical state of a substance? 12.4 Compare solids to liquids to gases with respect to internal order and distance between particles. 12.5 How do the physical properties of a liquid and solid reflect the different ordering of the particles present? 12.6 (a) Why do the densities of gases tend to be much less than those of liquids and solids? (b) Why would you expect the densities of a substance to be similar in the liquid and solid state? 12.7 What is a phase diagram? 12.8 What is the triple point on a phase diagram? 12.9 Define each of the following. (a) critical point (b) critical temperature (c) critical pressure 12.10 Why is the solid-liquid line on phase diagrams nearly vertical, while the solid-gas and liquid-gas lines show significant variation? 12.11 Explain why the liquid-gas line on a phase diagram ends at the critical point. 12.12 Sketch a general phase diagram. (a) Label the triple point. (b) Label the critical point. 12.13 Sketch a general phase diagram. (a) Indicate on your diagram where melting is important. (b) Indicate on your diagram where vaporization is important. (c) Indicate on your diagram where deposition is important. Figure 12.XXX Simplified phase diagram for CO2 see problem 12.14 © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 96 12.14 Answer the following questions by consulting Figure 12.XXX. (a) A sample of carbon dioxide, initially at – 56 °C and 4.0 atm is compressed at constant temperature to a pressure of 100 atm. What phase changes, if any, occur? (b) Another sample of carbon dioxide, initially at –80 °C and 10 atm is heated at constant pressure to 40 °C. What phase changes, if any, occur? (c) A third sample of carbon dioxide, initially at –100 °C and 4.0 atm, is heated at constant pressure to 25 °C. What phase changes, if any, occur? 12.15 Given the data below, construct a phase diagram for oxygen labeling each point and the solid, liquid, and gas regions. All temperatures are in °C, and all pressures are in atm. Temperature Pressure Triple point –218.80 0.015 Critical point –118.4 50.15 Normal melting point –218.79 Normal boiling point –187.97 Vapor pressure of solid –219.1 0.013 12.16 Given the data below, construct a phase diagram for radon, labeling each point and the solid, liquid, and gas regions. All temperatures are in °C, and all pressures are in atm. Temperature Pressure Triple point –71 0.658 Critical point 105 62 Normal melting point –71 Normal boiling point –62 Vapor pressure of solid –75 0.526 12.17 Two pans of water are placed on a stove and the heat is turned on high. One pan contains 1 L of water and the other contains 2 L of water. (a) If both pans are heated at the same rate, which will boil first? (b) If the water in the pan with less water is boiling at 100°C, what is the temperature of boiling water in the other pan? (c) Once both pans begin to boil, the heat is turned down on one pan so that it is barely boiling, while the other is left on high and boils vigorously. How do the temperatures of the water in the two pans compare? 12.2 Corresponds to BLBMWS Sections 11.1-11.2 12.18 (a) What are intermolecular forces? (b) List the types of intermolecular forces. 12.19 What are London dispersion forces? 12.20 What is an instantaneous dipole? 12.21 (a) What is the strongest type of intermolecular force that may be present between two nonpolar molecules? (b) What is the strongest type of intermolecular force that may be present between two polar molecules? 12.22 Where do dipole-dipole forces occur? 12.23 What types of molecules have dipole moments? 12.24 (a) What is hydrogen bonding? (b) What are the requirements for a hydrogen bond to be present? 12.25 List the different types of van der Waal's forces. 12.26 List the different types of van der Waals forces in order from weakest to strongest. 12.27 One member of your study group explains that a substance melts because the intermolecular forces are weaker in a liquid than in a solid. Explain what is wrong with this statement. You may want to use ice and liquid water in your discussion. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 97 12.28 (a) In what type of material is ionic bonding the strongest intermolecular force? (b) In what type of material is covalent bonding the strongest intermolecular force? (c) In what type of material is metallic bonding the strongest intermolecular force? 12.29 (a) Give three examples of substances where ionic bonding is the strongest intermolecular force. (b) Give three examples of substances where covalent bonding is the strongest intermolecular force. (c) Give three examples of substances where metallic bonding is the strongest intermolecular force. 12.30 List the type of intermolecular force that must be overcome in each of the following cases. (a) boiling liquid bromine (Br2) (b) melting iron (Fe) (c) melting solid sulfur dioxide (SO 2) (d) vaporizing graphite (C) (e) subliming ice 12.31 Choose the member of each pair that you expect to have the higher melting point. Explain your reasoning in each case. (a) aluminum fluoride (AlF3) or phosphorus trifluoride (PF3) (b) xenon (Xe) or argon (Ar) (c) hydrogen fluoride (HF) or hydrogen chloride (HCl) (d) sodium fluoride (NaF) or calcium oxide (CaO) (e) carbon dioxide (CO2) or silicon dioxide (SiO2) 12.3 Corresponds to BLBMWS Sections 11.3 and 11.5 12.32 (a) What is surface tension? (b) What happens to the surface tension if the intermolecular forces increase in strength? 12.33 (a) What is viscosity? (b) What happens to the viscosity if the intermolecular forces increase in strength? 12.34 (a) What is vapor pressure? (b) What happens to the vapor pressure if the intermolecular forces increase in strength? 12.35 Define capillary action. Use one or more diagrams to illustrate your definition. 12.36 How does the viscosity of a liquid vary with temperature? 12.37 What label refers to a liquid with such a high viscosity that it appears to be a solid? 12.38 What is one way of differentiating between a true solid and an amorphous solid? 12.39 Why is it not possible for an amorphous solid to melt? 12.40 What is a dynamic equilibrium? 12.41 State Le Châtelier’s principle. 12.42 (a) Define boiling point. (b) Define normal boiling point. 12.43 The following observations have been made concerning these liquids. Explain each. (a) The surface of mercury in a narrow glass tube is concave-down, but the surface of water, in the same tube, is concave-up. (b) Phosphorus trichloride (PCl3) has a higher surface tension than phosphorus pentachloride (PCl 5). 12.44 The following observations have been made concerning liquids. Explain each. (a) Liquid ammonia (NH 3) has a higher viscosity than liquid phosphine (PH3). (b) Oil in an automobile engine flows faster as the engine warms. 12.45 Explain how an increase in the intermolecular forces would affect each of the following properties. (a) surface tension (b) melting point (c) viscosity (d) heat of condensation (e) vapor pressure © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 98 Figure 12.XXX Plot of vapor pressure or water as a function of temperature. See question 12.47. 12.47 Atmospheric pressure at the top of Mount Everest is about 240 mmHg. Use Figure 12.XXX to estimate the boiling point of water at the top of Mount Everest. 12.48 Chloroform, CHCl3, was one of the first anesthetics. Other, safer, compounds are now used in place of chloroform. The normal boiling point of chloroform is 61.7°C and the heat of vaporization for this compound is 31.4 kJ/mole. Calculate the vapor pressure in torr of chloroform at 45.7°C. 432 torr 12.49 Sulfur dioxide melts at –73 °C and boils at –10. °C. The enthalpy of fusion of sulfur dioxide is 8.619 kJ/mol, and its enthalpy of vaporization is 25.73 kJ/mol. The specific heats of liquid and gaseous sulfur dioxide are 0.995 J/g•K and 0.622 J/g•K, respectively. How much heat is required to convert 2.50 kg of solid sulfur dioxide at the melting point to the vapor phase at 60. °C? 12.50 Hydrogen iodide melts at –51 °C and boils at –35 °C. The enthalpy of fusion of hydrogen iodide is 2.871 kJ/mol, and its enthalpy of vaporization is 44.11 kJ/mol. The specific heats of liquid and gaseous hydrogen iodide are 0.365 J/g•K and 0.228 J/g•K, respectively. How much heat is required to convert 220.0 g of solid hydrogen iodide at the melting point to the vapor phase at 0.0 °C? 12.4 Corresponds to BLBMWS Sections 12.1-12.3 and 12.5 12.51 (a) Define lattice. (b) How does a lattice relate to a crystalline solid? 12.52 (a) What is a unit cell? (b) How does a unit cell relate to a lattice? 12.53 What are the six parameters used to define a unit cell? 12.54 (a) Sketch a simple cubic unit cell. (b) Sketch a body-centered cubic unit cell. (c) Sketch a face-centered cubic unit cell. 12.55 What contribution toward the contents of a unit cell do atoms in the following positions supply? (a) corner (b) body-center (c) face-center (d) edge 12.56 (a) What is a closest packed structure? (b) What is the difference between a cubic closest packed structure and a hexagonal closest packed structure? (c) What is the coordination number of each atom in a closest packed structure? (a) The most efficient way of packing equal spheres together. (b) The difference is the arrangement of the layers. Every other layer is the same in hexagonal closest packed, while there is a three-layer sequence in cubic closest packed. (c) 12 12.57 Which type of cubic unit cell is the same as a cubic closest packed structure? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 99 Figure 12.XXX A comparison of the three types of cubic structures for problem 12.58. 12.58 Examine Figure 12.XXX. Calculate the number of atoms (spheres) present in (a) a simple cubic unit cell, (b) a body-centered cubic unit cell, (c) a face-centered cubic unit cell. 12.59 The unit cell pictured below is one view of the perovskite structure. The name for this type of structure is derived from the first substance determined to have this structure. Many ionic materials adopt this structure. The different types of atoms present are designated A, B, and X. A X B (a) Determine the formula for the cell contents of the perovskite structure. (Report the formula with the atoms in alphabetical order.) (b) Is this a primitive, body-centered, or face-centered structure? 12.60 The platinum(II) sulfide structure is shown below. The approximate geometry around the platinum atoms is square planar, as indicated by the dashed lines. The geometry about the sulfur atoms is tetrahedral. The cell is tetragonal with a height of 12.220 Å, and the other two dimensions are 3.470 Å. (a) How many PtS formula units are present in the unit cell? (b) Determine the density of platinum(II) sulfide in g/cm3. (a) 4 PtS (b) 10.25 g/cm3 12.61 Gold crystallizes with a face-centered cubic unit cell. Gold atoms may be treated as spheres with a radius of 1.44 Å. (a) Calculate the number of gold atoms in a unit cell. (b) Each gold atom is coordinated by how many other gold atoms? (c) Calculate the length of a unit-cell edge. (d) Determine the density of gold in g/cm3. (a) Four (b) Twelve (c) 4.07 Å (d) 19.4 g/cm3 © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 100 Figure 12.XXX The sodium chloride structure for problems 12.62 and 12.63. 12.62 Barium oxide (BaO), like many compounds, adopts the sodium chloride structure (see Figure 12.XXX). The unit cell edge is 3.90 Å. What is the density of barium oxide in g/cm 3? 12.63 The uncommon mineral bunsenite (NiO) adopts the sodium chloride structure (see Figure 12.XXX). The density of the mineral is 6.806 g/cm3. Determine the length of a unit-cell edge. 12.64 Ultrapure silicon may be used to calculate very accurate values of Avogadro's number. Silicon is cubic with a unit cell edge of 5.430940 Å. The unit cell contains eight silicon atoms with an atomic mass of 28.0855 amu. The density of silicon is 2.329143 g/cm3. Determine the value of Avogadro's number. 12.65 The density of tantalum (Ta) metal is 16.634 g/cm3. It is body-centered cubic with a cell edge of 3.3058 Å. From these data, calculate Avogadro's number. 12.66 Calculate the density of iron, in g/cm3, if it forms in a body-centered lattice with a cell edge of 2.8664 Å. 7.8752 g/cm3 12.67 Iron has a density of 7.8752 g/cm3. The edge of a body-centered cubic iron unit cell is 2.8664 Å. Using this information, calculate Avogadro’s number. 6.02 × 1023 Fe atoms/mol 12.68 Calculate Avogadro's number given that potassium metal is body-centered cubic with a unit cell edge of 5.103 Å. The density of potassium is 0.977 g/cm3. 12.69 Lithium adopts a body-centered cubic lattice with a cell edge of 3.5100 Å. Determine the radius of a lithium atom. 12.70 A metal with a density of 0.966 g/cm3 adopts a body-centered cubic lattice. The lattice edge is 4.2908 Å. Determine the atomic weight of the metal. 12.71 A sample of an unknown metal has a density of 5.250 g/cm3. X-ray diffraction studies show the metal to be body-centered cubic with a unit cell edge of 458.1 pm. Determine the atomic weight and identify the element. 12.72 Tungsten metal has a body-centered cubic unit cell. The density of tungsten is 19.3 g/cm3. The tungsten atoms touch each other along the body diagonal of the unit cell. Determine the atomic radius of tungsten atoms in pm. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 101 Figure 12.XXX The cesium chloride structure for problem 12.73. 12.73 Cesium iodide (CsI) like many compounds adopts the cesium chloride structure (see Figure 12.XXX). The unit cell edge is 4.57 Å. What is the density of cesium iodide in g/cm 3? 12.74 Calculate Avogadro's number given that copper metal is face-centered cubic with a unit cell edge of 3.6150 Å. The density of copper is 8.96 g/cm3. 12.75 Silver adopts a face-centered cubic lattice with a cell edge of 4.0862 Å. Determine the radius of a silver atom. 2.0431 Å 12.76 A metal with a density of 21.472 g/cm3 adopts a face-centered cubic lattice. The lattice edge is 3.9231 Å. Determine the atomic weight of the metal. 12.77 Cesium metal has a body-centered cubic unit cell. The density of cesium is 1.90 g/cm3. The cesium atoms touch each other along the body diagonal of the unit cell. Determine the atomic radius of cesium atoms in pm. 12.5 Corresponds to BLBMWS Sections 12.1-12.2 and 12.6-12.8 12.78 What are the four categories of solids? 12.79 Summarize the properties of an ionic solid. 12.80 Summarize the properties of a network solid. 12.81 Summarize the properties of a metallic solid. 12.82 Summarize the properties of a molecular solid. 12.83 Why may a network solid also be called a covalent solid? 12.84 Why do chemists group atomic solids, such as solid xenon, with molecular solids? 12.85 Predict the type of crystal each of the following substances is expected to form. (a) water (H 2O) (b) silicon dioxide (SiO2) (c) uranium (U) (d) carbon dioxide (CO 2) (e) ammonium nitrate (NH4NO3) 12.86 Indicate the type of crystal each of the following would form upon solidification. (a) Carbon dioxide (b) Silicon dioxide (c) Calcium chloride (d) Hydrogen fluoride (e) Silver 12.87 Which solid in each pair would be expected to have a higher melting point? (a) water (H 2O) or hydrogen sulfide (H2S) (b) carbon tetraiodide (CI4) or carbon tetrabromide (CBr4) (c) carbon (C) or acetic acid (HC2H3O2) (d) ethylene glycol (CH2OHCH2OH) or sodium chloride (NaCl) (e) hydrogen fluoride (HF) or barium (Ba) 12.88 A bright yellow solid melts at 968 °C. The solid does not conduct electricity, but an aqueous solution of the solid will conduct electricity. This material is most likely to be which type of solid? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 102 12.6 Summary 12.89 (a) Draw the Lewis structures for water, ammonia, and methane. (b) What two factors, lacking in methane, make water and ammonia good solvents? 12.95 Sketch how a single water molecule can form part of four hydrogen bonds. 12.101 What is the cost advantage of "high-temperature" superconductors? 12.102 One of the first materials to be termed a "high-temperature" superconductor was the ceramic YBa2Cu3O7. (a) How does this ceramic differ from previously known superconductors? (b) Determine the average oxidation state of copper. Assume that all the other elements adopt their normal oxidation states. (c) Related ceramics, where the Y or Ba atoms are replaced with other materials, are superconductors unless the copper is replaced. Write the electron configurations for all the ions in this compound. How does the electron configuration of copper differ from the configurations of other elements? 12.7 Summary 12.103 Rank the following compounds in order of decreasing vapor pressure. (a) CH 3CH2CH2CH3 (b) CH3CH2CH2OH (c) CH3OCH2CH3 (d) HOCH2CH2OH 12.104 (a) From the following list, choose the compounds that exhibit hydrogen bonding as the strongest intermolecular force present: nitric acid (HNO3), stibine (SbH3), methane (CH4), sodium hydroxide (NaOH), dimethylamine ((CH3)2NH). (b) Choose the substances from the following list that exhibit dipole-dipole attractions as the strongest type of intermolecular force present: hydrogen chloride (HCl), silicon dioxide (SiO 2), methanol (CH3OH), sulfur tetrafluoride (SF4), krypton difluoride (KrF2) 12.105 Each of the following is inhibited by either an intramolecular or an intermolecular force; in each case, decide which type of force is involved. (a) A chlorine molecule (Cl 2) separates into chlorine atoms. (b) Solid sodium chloride (NaCl) melts at 25°C. (c) Iron (Fe) rusts to give FeO(OH). (d) Water spreads out evenly on a waxed surface. (e) A diamond vaporizes while setting in a jeweler's display case. 12.106 Molybdenum metal has a body-centered cubic unit cell. The density of molybdenum is 10.2 g/cm3. (a) Determine the edge of a unit cell of molybdenum. (b) The molybdenum atoms touch each other along the body diagonal of the unit cell. Determine the atomic radius of molybdenum atoms. 12.107 Silicon carbide (SiC) has a three-dimensional structure similar to that of diamond. If one-half of the carbons are replaced with silicon atoms, a structure results where each carbon is tetrahedrally surrounded by silicon atoms, and carbon atoms tetrahedrally surround each silicon atom. Silicon carbide has many applications in industry because it is very hard, nearly as hard as diamond, and has a very high melting point. What type of solid (molecular, ionic, covalent, or metallic) is silicon carbide? 12.108 It is possible to cool water by evaporation; this is especially effective in the desert. The evaporation of water from the outside surface of a closed container will cool the water inside the container. What volume of water, in milliliters, can the evaporation of 25.0 g of water cool from 45.0 °C to 20.0 °C? The heat of vaporization of water is 40.7 kJ/mol, the specific heat of water is 4.18 J/g°C, and the density of water is 1.00 g/mL. 12.109 The normal boiling point of ethyl alcohol is 78.4°C. The heat of vaporization of this compound is 40.5 kJ / mol. Calculate the vapor pressure, in atmospheres, of ethyl alcohol at a temperature of 55.0°C. 12.110 The normal boiling point of acetone is 56.1°C. The heat of vaporization of this compound is 29.1 kJ / mol. Calculate the vapor pressure, in atmospheres, of acetone at a temperature of 25.0°C. 0.330 atm 12.111 The vapor pressure of diethyl ether at 25.0°C is 545 torr. The heat of vaporization of this compound is 26.5 kJ / mol. Calculate the normal boiling point, in degrees Celsius, of diethyl ether. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 103 12.112 What type of phase transition occurs during each of the following changes: (a) dew forms on grass (b) ice cubes in a freezer slowly disappear (c) when warmed in a pan of a stove, butter changes to a liquid (d) water in a glass slowly disappears (e) gaseous carbon dioxide forms dry ice (solid carbon dioxide). Chapter 13 13.1 Corresponds to BLBMWS Section 13.1 13.1 Give an example of each of the following types of solution. (a) a solid solute in a liquid solvent (b) a liquid solute in a liquid solvent (c) a gaseous solute in a liquid solvent (d) a gaseous solute in a gaseous solvent (e) a solid solute in a solid solvent 13.2 (a) List the general characteristics of a homogeneous mixture. (b) List the general characteristics of a heterogeneous mixture. 13.3 (a) Which process(es) during dissolution require energy? (b) Which process(es) during dissolution release energy? 13.4 What does the phrase "like dissolves like" mean? 13.5 Oil and water do not mix. What conclusions may be made about the types of intermolecular forces in oil and water? 13.6 Why is a nonpolar substance like oxygen capable of dissolving in a very polar solvent like water? 13.7 Ionic bonding is a very strong type of intermolecular force. Why is water capable of overcoming this force in some cases? 13.8 Sodium chloride will dissolve in water. What would be the general characteristics of another solvent that might dissolve sodium chloride? 13.9 When sodium sulfate (Na2SO4) dissolves in water, what type(s) of intermolecular forces break, and what kind(s) form? 13.10 Which of the following solvents are polar, and which are nonpolar? (a) methanol (CH 3OH) (b) carbon tetrachloride (CCl4) (c) ammonia (NH3) (d) bromine trifluoride (BrF3) (e) ethane (C2H6) 13.11 Determine the most important type of solvent-solute interaction in each of the following. (a) iodine (I 2) dissolved in carbon tetrachloride (CCl4) (b) acetone, (CH3COCH3) dissolved in water (c) sodium chloride (NaCl) dissolved in water (d) ethanol (CH3CH2OH) dissolved in water (e) manganese (Mn) dissolved in iron (Fe) 13.12 Explain which member from each of the following pairs is more soluble in water (a) chloroform (CHCl 3) or aluminum chloride (AlCl3) (b) methanol (CH3OH) or methane (CH4) (c) diethyl ether, (CH3CH2)2O, or ethanol, CH3CH2OH (d) ammonia, (NH3) or phosphine (PH3) (e) lead(II) sulfide (PbS) or sodium sulfide (Na 2S) 13.13 Define heat of solution and tell what factors influence its value. 13.14 Define the following terms. (a) hydrated ion (b) hydration energy (c) hydrate (d) water of hydration 13.2 Corresponds to BLBMWS Section 13.2-13.3 13.15 Define the following terms as they apply to solutions. (a) solubility (b) saturated (c) unsaturated (d) supersaturated 13.16 (a) How does a change in temperature affect the solubility of most solids? (b) How does a change in temperature affect the solubility of gases? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 104 13.17 (a) How does a change in pressure affect the solubility of most solids? (b) How does a change in pressure affect the solubility of gases? 13.18 What law applies to the solubility of gases? Figure 13.XXX Solubilities of some ionic compounds in water as a function of temperature. See question 13.19. 13.19 Examine Figure 13.XXX. A series of solutions, one for each compound in the figure, are prepared by adding 50.0 g of solute to 100.0 g of water. The solutions are heated to 90°C to help the solutes dissolve faster. In which cases, will all the solute fail to dissolve completely? 13.3 Corresponds to BLBMWS Section 13.4 13.20 Define the following concentration units. (a) molarity (b) mole fraction (c) weight percent (d) volume percent (e) molality 13.21 Which concentration unit always considers the solute and solvent separately and never combined? 13.22 (a) What units may be used in calculating the weight percent? (b) What units may be used in calculating the volume percent? 13.23 Calculate the mass percent of solute in each of the following solutions. (a) 25.0 g of sodium chloride (NaCl) in 1000.0 g of water (b) 0.25 mol of nitric acid (HNO 3) in 100.0 g of water 13.24 Calculate concentration in parts per million (ppm) of solute in each of the following solutions (a) 0.32 g of chloride ion (Cl−) in 100.0 g of solution (b) 1.3 × 10 −3 g of lead(II) ions (Pb2+) in 1.00 kg of water (a) 3.2 × 103 ppm (b) 1.3 ppm 13.25 Calculate the mole fraction of solute in each of the following solutions. (a) 1.2 mol of ethanol (CH 3CH2OH) in 5.2 mol of water (b) 0.55 mol of acetone (CH3COCH3) in 250.0 g of water (c) 15.5 g of iodine (I 2) dissolved in 275 g of carbon tetrachloride (CCl4). 13.26 Calculate the molarity of solute in each of the following solutions: (a) 0.62 mol of potassium bromide (KBr) dissolved in 0.750 L of solution (b) 3.75 g of ammonium nitrate (NH 4NO3) dissolved in 0.500 L of solution (c) 5.52 g of copper(II) sulfate pentahydrate (CuSO4·5H2O) dissolved in 675 mL of solution. (a) 0.83 M KBr (b) 0.0937 M NH4NO3 (c) 0.0328 M CuSO4•5 H2O 13.27 Calculate the molarity of each type of ion in each of the following solutions: (a) 0.75 mol of sodium sulfate (Na2SO4) dissolved in 0.650 L of solution (b) 14.3 g of potassium phosphate (K 3PO4) dissolved in 2.50 L of solution (c) 2.53 g of iron(III) bromide hexahydrate (FeBr 3·6H2O) in 875 mL of solution. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 105 13.28 Calculate the molarity of solute in each of the following solutions: (a) 5.3 g of bromine (Br 2) dissolved in sufficient chloroform (CHCl3) to prepare 250 mL of solution (b) 14.7 g of diethyl ether ((C 2H5)2O) dissolved in sufficient ethyl alcohol (C2H5OH) to prepare 500. mL of solution (c) 35.2 g of 2-propanol (CH3CHOHCH3) dissolved in sufficient water to prepare 100.0 mL of solution. (a) 0.13 M Br2 (b) 0.397 M (C2H5)2O (c) 5.86 M Br2 13.29 A phosphoric acid (H3PO4) solution was prepared by dissolving 935 g of phosphoric acid in sufficient water to prepare 1.00 L of solution. The density of this solution is 1.461 g/cm3. (a) What is the mass percentage of phosphoric acid in the solution? (b) What is the mole fraction of phosphoric acid in the solution? (c) Determine the molality of the phosphoric acid. (d) Calculate the molarity of the phosphoric acid. 13.30 Concentrated hydrofluoric acid (HF) has 40.0 mass percent HF in an aqueous solution. The density of the solution is 1.128 g/cm3. (a) Calculate the molarity of hydrofluoric acid in the solution. (b) What is the molality of this solution? 13.31 Concentrated nitric acid has 68.0 mass percent acid in an aqueous solution. The density of the solution is 1.41 g/cm3. (a) Calculate the molarity of nitric acid in the solution. (b) What is the molality of this solution? (a) 15.2 M HNO3 (b) 33.7 m HNO3 13.32 How many moles of solute are present in each of the following: (a) 750.0 mL of 2.1 M ammonium nitrate (NH4NO3) (b) 275 mL of a solution containing 71.8 g of acetic acid (HC2H3O2) in 153 g of water if the density of the solution is 1.055 g/cm3 (c) 25.0 g of a solution that is 5.85 mass percent sodium hydroxide (NaOH). 13.33 Describe how to prepare each of these solutions using commonly available laboratory apparatus. (a) 100.0 g of a 10.0 % potassium bromide (KBr) solution, made from the solid solute and water (b) 100.0 mL of 0.500 M sodium chloride (NaCl) made from solid solute and water (c) a solution with the mole fraction of ethylene glycol (C2H6O2) equal to 0.275, made from liquid ethylene glycol and water, with 1.00 mole of ethylene glycol present (d) a solution containing 0.100 mol of ammonium ion in 250.0 mL of solution, made from solid ammonium phosphate, (NH4)3PO4, and water 13.34 Oxalic acid (H2C2O4) and its salts are present in plants such as spinach and rhubarb. A 0.580-m oxalic acid aqueous solution has a density of 1.022 g/mL. (a) What is the molarity of this solution? (b) What is the mass percent of oxalic acid in this solution? 13.35 Calculate the molarity of a solution made by dissolving 0.17500 g of CuCr 2O7•2H2O in enough water to make 1250.0 mL of solution. 4.4365 × 10–4 M 13.4 Corresponds to BLBMWS Section 13.5 13.36 What are colligative properties? 13.37 How does the presence of a nonvolatile solute affect the vapor pressure of a solution? 13.38 What is an ideal solution? 13.39 What experimental data is necessary to determine if a solution is ideal? 13.40 (a) If a solution is ideal, what can be said about the relative intermolecular forces of the solute and solvent? (b) If a solution shows a positive deviation from Raoult's law, what can be said about the relative intermolecular forces of the solute and solvent? (c) If a solution shows a negative deviation from Raoult's law, what can be said about the relative intermolecular forces of the solute and solvent? 13.41 (a) A solution is prepared by mixing 175 g of chloroform (CHCl 3) with 275 g of carbon tetrachloride (CCl4). The vapor pressure of pure chloroform is 375 torr. The vapor pressure of pure carbon tetrachloride is 143 torr. What is the vapor pressure of the solution? (b) If the observed vapor pressure of the solution were 215 torr, would the solution be ideal or show a positive or negative deviation? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 106 13.42 What is the vapor pressure of a solution made by adding 25.0 g of glucose, (C 6H12O6) to 375 g of water at 80°C? Glucose is not volatile, and the vapor pressure of water at 80°C is 355 torr. 13.43 (a) At 40°C, acetone (CH3COCH3) has a vapor pressure of 4.00 × 10 2 mmHg. Glycerol (C3H8O3) has a negligible vapor pressure at this temperature. How many grams of glycerol must be added to 750.0 g of acetone to produce a solution with a vapor pressure 15 mmHg lower than that of pure acetone? (b) At 40°C, water has a vapor pressure of 55.3 torr. Calcium chloride (CaCl2) has a negligible vapor pressure at this temperature. How many grams of calcium chloride must be added to 1.00 kg of water to produce a solution with a vapor pressure 7.5 torr lower than that of pure water? 13.44 A solution made by dissolving 36.6 g of a nonvolatile substance in 242.7 g of CHCl 3 has a vapor pressure of 411 mmHg. At the same temperature, the vapor pressure of pure CHCl3 is 526 mmHg. Determine the molecular weight of the substance. 64.3 g/mol 13.45 A solution of 96.6 g of an unknown substance in 800.0 g of carbon tetrachloride has a vapor pressure of 143 mmHg. The vapor pressure of the pure unknown is 85 torr, and the vapor pressure of pure carbon tetrachloride is 157 mmHg (all vapor pressures are determined at 30°C). Determine the molecular weight of the unknown substance. 13.5 Corresponds to BLBMWS Section 13.5 13.46 Which colligative property is each of the following descriptions of concentration used to calculate? (a) molarity (b) molality (c) mole fraction 13.47 What is the van't Hoff factor? 13.48 Rank the following 0.100-m solutions in order of decreasing melting point. (a) sodium chloride (NaCl) (b) sucrose (table sugar; C12H22O11) (c) aluminum nitrate, Al(NO3)3 (d) barium chloride (BaCl2) 13.49 Under what circumstances may the van't Hoff factor not be a whole number? 13.50 What is a semi-permeable membrane? 13.51 Define osmosis and osmotic pressure. 13.52 What is reverse osmosis? 13.53 Which of the following are colligative properties of solutions? (a) osmotic pressure (b) mass percent (c) density (d) vapor pressure (e) boiling point 13.54 Use the data in the table below to determine the boiling and freezing points of the following solutions: (a) 0.75 m iodine (I2) in benzene (C6H6) (b) 5.3 g white phosphorus P 4) in 175 g of carbon disulfide (CS2) (c) 3.5 g of ammonium sulfate, (NH4)2SO4, in 175 mL of water. The density of water is 1.00 g/mL. Solvent melting Boiling Kf Kb point (°C) point (°C) (°C/m) (°C/m) Water 0.00 100.00 1.86 0.512 Benzene 5.5 80.1 4.90 2.53 Carbon disulfide –111.5 46.2 3.83 2.34 13.55 Palmitic acid, from palm oil, is a constituent of saturated fats that is useful in making some soaps. A 2.50-g sample of palmitic acid is dissolved in 0.150 kg of cyclohexane (C6H12). The freezing point of the solution was 5.25°C, as opposed to 6.55°C for pure cyclohexane. The freezing point depression constant for cyclohexane is 20.0 °C/m. Calculate the molar mass of palmitic acid. 13.56 A solution made by dissolving a 1.00 g sample of iron chloride 100.0 mL of water has a freezing point of – 0.46°C. What is the formula of the compound? © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 107 13.57 A solution made by dissolving 0.100 g of a polymer (very large molecule) in 1.50 g of carbon tetrachloride has a freezing point depression of 0.61°C. If the freezing point depression constant of carbon tetrachloride is 29.8°C/m, what is the molar mass of the polymer? 13.58 A solution contains 1.20 g of an organic acid in 75.0 g of cyclohexane. This solution has a freezing point that is 1.50°C lower than that of pure cyclohexane. The freezing point depression constant, K f, for cyclohexane is 20.0°C/m. Determine the molar mass of this acid. 13.59 Beeswax contains a number of waxes. Hydrolysis of beeswax results in a variety of compounds. One of the products, isolated through a chromatographic procedure, is soluble in cyclohexane. A total of 1.25 g of this compound dissolves in 60.0 g of cyclohexane to produce a solution with a melting point 1.25°C lower than pure cyclohexane. The freezing point depression constant, Kf, for cyclohexane is 20.0°C/m. Determine the molar mass of this compound. 13.60 You are given three aqueous solutions: 0.80 m sodium chloride (NaCl) 0.80 m methanol (CH3OH) 0.60 m calcium nitrate (Ca(NO3)2). Rank the solutions in order of increasing freezing point depression. 13.61 For water, melting point = 0.00°C; boiling point = 100.00°C; freezing point depression constant = 1.86°C/m; boiling point elevation constant = 0.512°C/m. Determine the freezing point for each of the following. (a) 0.200 m ammonium nitrate (NH4NO3) (b) 0.200 m ammonium chromate ((NH4)2CrO4) 13.62 What is the boiling point of an aqueous solution with a freezing point of −7.35°C? 13.63 A cyclohexane (solvent) solution boils at 83.20°C; what is the freezing point of this solution? The normal freezing and boiling points of cyclohexane are 6.55°C and 80.74°C, respectively. The freezing point depression constant, Kf, for cyclohexane is 20.0°C/m and the boiling point elevation constant is 2.79 °C/m. 13.64 Determine the freezing point of a solution containing 200.0 g of cobalt(II) perchlorate in 200.0 g of water. 13.65 Determine the freezing point of a solution containing 101.50 g of magnesium bromide in 200.0 g of water. 13.66 For water, melting point = 0.00°C; boiling point = 100.00°C; freezing point depression constant = 1.86°C/m; boiling point elevation constant = 0.512°C/m. Determine the freezing point of a solution containing 3.04 g of ammonium sulfate in 100.0 g of water. 13.67 A solution prepared by dissolving, in 1000.0 mL of water, 0.432 g of compound extracted during the autopsy of a patient’s brain has an osmotic pressure of 7.40 mmHg at 25°C. Calculate the molecular weight of this hormone. 1.08 × 103 g/mol 13.68 A solution prepared by dissolving 6.850 g of a carbohydrate in 100.0 g of water has a density of 1.024 g/mL, and at 20.0 °C, the osmotic pressure is 4.61 atm. Calculate the molecular weight of the carbohydrate. 13.69 A solution of a protein contains 0.382 g of the material in 0.500 mL of solution. At a temperature of 25°C, this solution has an osmotic pressure of 2.07 torr. Calculate the molar mass of the protein. 13.70 A liter of blood contains about 150 g of hemoglobin. In order to determine the molecular weight of hemoglobin, a solution was prepared by dissolving 150.0 g of hemoglobin in enough water to produce 1.000 L of solution. The resultant solution had an osmotic pressure of 0.056 atm at 25°C. Calculate the molecular weight of hemoglobin. 6.6 × 104 g/mol 13.71 A 0.0100 M solution of potassium sulfate (K2SO4) has an osmotic pressure of 5.0 × 10 2 torr at 25°. (a) Estimate the van't Hoff factor for this solution based on the chemical formula of potassium sulfate. (b) Determine the actual van't Hoff factor for this solution. (c) Why do the values in parts (a) and (b) not agree? 13.72 Determine the molecular weight of a protein if a solution, prepared by dissolving 3.320 g of protein in enough water to make 340.0 mL of solution, has an osmotic pressure of 10.40 mmHg at 25.0°C. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 108 13.73 The primary solute in seawater is sodium chloride (NaCl). There are approximately 27 g of sodium chloride dissolved in every kilogram of seawater. The density of seawater is 1.0250 g/mL. Calculate the osmotic pressure generated by the sodium chloride in seawater at 25°C. 13.74 Determine the molecular weight of a starch sample if a solution with a volume of 200.0 mL has 1.596 g of starch dissolved in it. The osmotic pressure of the solution is 4.58 torr at 25°C. 13.75 The hormone vasopressin causes, among other things, a reduction in the excretion of water. A solution of this hormone is prepared by dissolving 0.200 g of this compound in sufficient water to prepare 250.0 mL of solution. At 30.°C, the solution has an osmotic pressure of 13.9 torr. Calculate the molar mass of vasopressin. 13.76 A solution of a polymer contains 0.400 g of material in 1.00 L of water. This solution has an osmotic pressure of 2.14 torr at 27°C. Calculate the molecular weight of the polymer. 13.77 A solution made by dissolving 0.500 g of insulin in 500.0 mL of water has an osmotic pressure of 3.24 torr at 25°C. Calculate the molecular weight of insulin. 13.78 Pepsin, an enzyme found in the stomach, is a protein that aids in digestion. A solution made by dissolving 0.600 g of pepsin in 1000.0 mL of water has an osmotic pressure of 0.324 torr at 25°C. Calculate the molecular weight of pepsin. 3.44 × 104 g/mol 13.6 Corresponds to BLBMWS Section 13.6 13.80 What is the Tyndall effect? 13.81 (a) How are a suspension and a colloid similar? (b) How are a solution and a colloid similar? 13.82 How might a beam of light be used to distinguish between a solution, a colloid, and a suspension? 13.83 Discuss similarities and differences between colloids and solutions. 13.84 Why, when viewed from an angle, does the light from a movie projector "glow" in a darkened theater? 13.7 Summary 13.89 Sodium stearate (NaC18H35O2) is a common constituent of many types of soap. This compound undergoes aerobic decomposition. (a) Write a balanced chemical equation for the reaction of this compound with oxygen (O 2) to yield carbon dioxide (CO2), water (H2O), and sodium ions (Na+), assume the solution is acidic. (b) How many grams of oxygen are necessary to oxidize 1.00 pounds of this soap? 13.8 Summary 13.95 Dialysis of blood is hemodialysis. The presence of blood cells requires a solution that is isotonic. A typical isotonic solution has the following quantities of solute dissolved in 100.0 mL of water: 0.60 g of sodium chloride (NaCl), 0.040 g of potassium chloride (KCl), 0.20 g of sodium bicarbonate (NaHCO 3), and 0.72 g of glucose (C6H12O6). (a) What is the molarity of each of the solutes, assuming the volume of the solution is equal to the volume of the solvent? (b) What is the osmotic pressure of this solution at body temperature (37 °C)? (Hint: Do not forget that some of the solutes are strong electrolytes.) 13.97 (a) Why is it useful to lower the freezing point of the liquid in an automobile cooling system? (b) Why is it useful to increase the boiling point of the liquid in an automobile cooling system? 13.98 Why is ethylene glycol better than methyl alcohol as antifreeze? (Hint: The vapor pressure of methyl alcohol is about 100 mmHg at room temperature, and the vapor pressure of ethylene glycol is less than 1 mmHg at this temperature.) © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 109 13.99 While it is less toxic than methyl alcohol, ethylene glycol (CH2OHCH2OH) is poisonous. The density of ethylene glycol is 1.1132 g/mL. A dog may die by ingesting 50 mL of ethylene glycol. How many moles of ethylene glycol are in 50.0 mL? 13.100 Equal volumes of water and ethylene glycol (CH2OHCH2OH) are mixed and added to an automobile radiator. The density of water is 0.99823 g/mL, and the density of ethylene glycol is 1.1132 g/mL. Calculate the boiling and freezing points of this solution. 13.101 An antifreeze solution is made by mixing 100.0 mL of water, density 1.0 g/mL, with 100.0 mL of ethylene glycol (CH2OHCH2OH), density 1.1 g/mL. The vapor pressure of water at 25°C is 24 torr, and the vapor pressure of ethylene glycol is negligible. (a) What is the vapor pressure of this solution at 25°C? (b) Assuming the volumes are additive, what is the osmotic pressure of this solution at 25°C? 13.9 Summary 13.102 Classify each of the following as a strong electrolyte, a weak electrolyte, or a nonelectrolyte in aqueous solution. (a) nitric acid (HNO3) (b) ethanol (C2H5OH) (c) chlorous acid (HClO2) (d) glucose (C6H12O6) (e) ammonia (NH3) 13.103 Blood has an osmotic pressure of 7.7 atm at 37°C. In cases where a patient needs more liquid in his or her blood, a saline solution (that is, a sodium chloride solution) may be administered intravenously. What must be done to the concentration of the saline solution to maintain the osmotic pressure of the blood? 13.104 Two solutions are prepared. The first contains 4.32 g of iodine (I 2) in 250.0 mL of carbon disulfide (CS2), and the second contains 4.32 g of iodine in 250.0 mL of carbon tetrachloride (CCl 4). The density of pure carbon disulfide is 1.263 g/mL, and the density of pure carbon tetrachloride is 1.589 g/mL. Assume the density of each solution is identical to that of the pure solvent. (a) Determine the mass percent of iodine in each solution. (b) Determine the mole fraction of iodine in each solution. (c) Determine the molality of iodine in each solution. (d) Determine the molarity of iodine in each solution. (e) Compare the two values in each case and comment on any similarities and differences. 13.105 Estimate the percent ionization of a 0.200 m aqueous solution of formic acid, (HCHO2) if the solution freezes at −0.383°C. 13.106 What is the name or the formula of each of the following compounds? (a) HOCN (b) H 2CrO4 (c) NH4Cl (d) CrAsO4 (e) CdCl2 (f) tin(II) oxide (g) thorium(IV) fluoride (h) radium oxide (i) krypton difluoride (j) sulfur trioxide 13.107 What is the name or the formula of each of the following compounds? (a) thiocyanic acid (b) HMnO 4 (c) (NH4)2HPO4 (d) radium(III) nitrate (e) XeF2 (f) silver(II) oxide (g) PbO (h) molybdenum(III) sulfate (i) tungsten(VI) oxide (j) As2O3 13.108 What is the name or the formula of each of the following compounds? (a) HClO 2 (b) HIO4 (c) Mn(ClO)3 (d) Cr2S3 (e) HgF2 (f) ammonium oxalate (g) plutonium(II) iodide (h) radon difluoride (i) chlorine trifluoride (j) potassium superoxide 13.109 What is the name or the formula of each of the following compounds? (a) oxalic acid (b) HCN (c) CuOCN (d) ammonium dichromate (e) UO2 (f) tin(II) thiosulfate (g) Pu(CO3)2 (h) chromium(II) phosphate (i) vanadium(IV) bicarbonate (j) N2O3 13.110 The cooling system of an automobile contains 3.00 gallons of water and 1.00 gallon of antifreeze (ethylene glycol, C2H6O2). The density of water is 8.34 lbs/gal, and the density of ethylene glycol is 9.29 lbs/gal. What is the freezing point of the solution in the automobile in °C? 13.111 Determine the molecular formula of a starch sample if a solution with a volume of 200.0 mL has 3.192 g of starch dissolved in it. The osmotic pressure of the solution is 9.16 torr at 25°C. The starch is 40.0 % carbon, 6.67 % hydrogen, and 53.3 % oxygen. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission. 110 13.112 When a 24.8-g sample of sodium nitrate dissolves in 500.0 g of water in a coffee-cup calorimeter, the temperature changes from 25.30 °C to 22.51 °C. Determine the heat of solution, in kJ/mol of sodium nitrate, for the dissolution of sodium nitrate in water. Assume that the specific heat of the solution is the same as that of pure water. 13.113 Complete and balance the equations for any reactions that occur when mixing the following aqueous solutions. Then write net ionic equations and identify the spectator ions. (a) nitric acid with calcium hydroxide (b) hydrochloric acid and lead(II) acetate (c) aluminum nitrate and barium hydroxide (d) ammonium sulfate and calcium nitrate (e) magnesium chlorate and potassium carbonate 13.114 Why is bromine (Br2) more soluble in carbon tetrachloride (CCl4) than iodine (I2) is? 13.115 For very dilute solutions, the molarity and molality are nearly equal. Assuming this is true, what is the freezing point of an aqueous solution of a nonelectrolyte with an osmotic pressure of 6.6 atm at 25°C? 13.116 Liquid mercury is capable of dissolving many substances to produce solutions known as amalgams. Which of the following substances are most likely to dissolve in liquid mercury? (a) NaCl (b) Na (c) Cl 2 13.117 A solution is prepared by dissolving 5.00 g of mercury(II) chloride in 500.0 g of water. The solution has a freezing point of −0.068°C. Is mercury(II) chloride a strong electrolyte, a weak electrolyte, or a nonelectrolyte? 13.118 Why will water vapor and carbon tetrachloride vapor form a solution when liquid water and liquid carbon tetrachloride will not? 13.119 You have a solution of potassium nitrate (KNO3) and a bottle of solid potassium nitrate. Devise an experiment to test the solution to determine if it is unsaturated, saturated, or supersaturated. Explain what you would see in each case. 13.120 Potassium permanganate (KMnO4) will dissolve in water. At 20°C, 100.0 mL of water will dissolve 6.38 g of potassium permanganate. At 65°C, 100.0 mL of water will dissolve 25 g of potassium permanganate. (a) How much potassium permanganate will dissolve in 50.0 mL of water at 65°C? (b) If the solution from part (a) cools to 20°C, what type of solution would be present if no potassium permanganate precipitates? (c) If the solution from part (a) cools to 20°C, what type of solution would be present if potassium permanganate precipitates? 13.121 Either sodium chloride (NaCl) or calcium chloride (CaCl2) may be used to de-ice a sidewalk. You have 1.00 pound of each of these compounds. To de-ice the sidewalk you will need to lower the freezing point of water to at least −10.0°C. Which of the two compounds will de-ice more sidewalks? 13.122 An adult male has about 7.0 L of blood. (a) Assuming the density of blood is the same as that of water, how many grams does the blood weigh? (b) A person is considered intoxicated if the alcohol (ethyl alcohol, C2H5OH) concentration in the blood is 0.080%. How many grams of alcohol are present in the bloodstream of a person with an alcohol concentration of 0.080%? (c) The density of alcohol is 0.79 g/mL. How many milliliters does your answer to part (b) represent? (d) The body is capable of absorbing about 22% of the alcohol a person drinks. How much total alcohol must a person drink to lead to the absorption of the volume calculated in part (c)? (e) Typical liquors are about 80 proof, which corresponds to 40% alcohol. How many milliliters of typical liquor must a person drink to ingest the volume of alcohol calculated in part (d)? 13.123 Determine the freezing point of a solution containing 65.0 g of aluminum sulfate in 200.0 g of water. © Sevagram Enterprises 2015 No part of this text may reproduced in any form without written permission.