Molar Mass and Percent Composition
advertisement
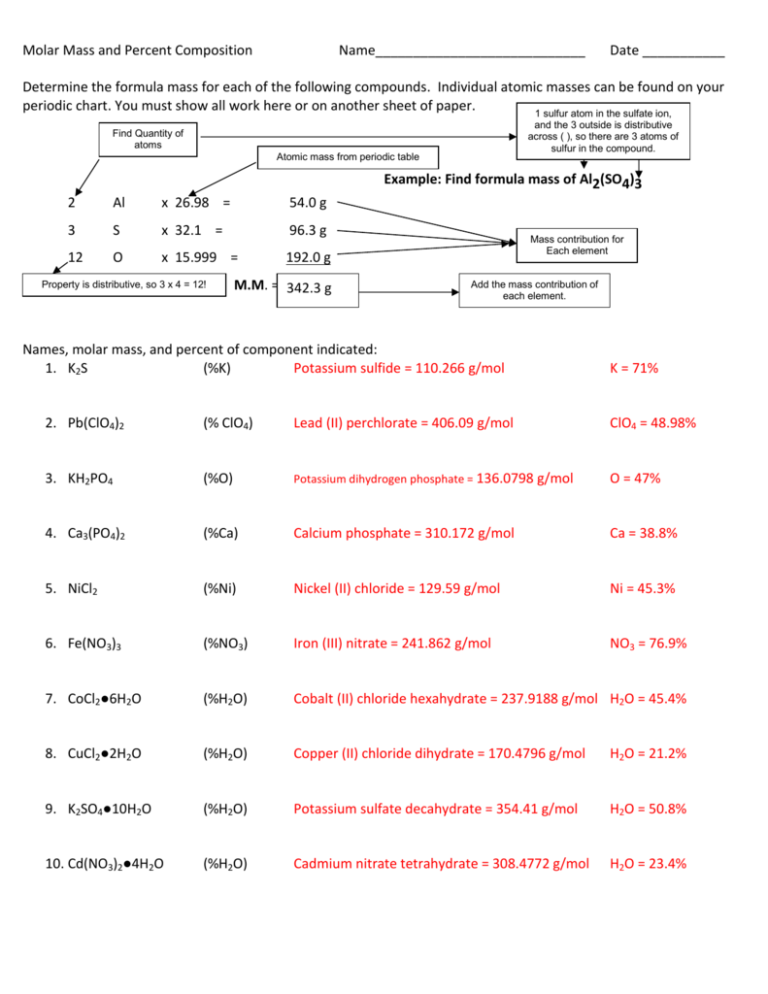
Molar Mass and Percent Composition Name____________________________ Date ___________ Determine the formula mass for each of the following compounds. Individual atomic masses can be found on your periodic chart. You must show all work here or on another sheet of paper. 1 sulfur atom in the sulfate ion, and the 3 outside is distributive across ( ), so there are 3 atoms of sulfur in the compound. Find Quantity of atoms Atomic mass from periodic table Example: Find formula mass of Al2(SO4)3 2 Al x 26.98 = 54.0 g 3 S x 32.1 = 96.3 g 12 O x 15.999 = 192.0 g Property is distributive, so 3 x 4 = 12! M.M. = 342.3 g amu Mass contribution for Each element Add the mass contribution of each element. Names, molar mass, and percent of component indicated: 1. K2S (%K) Potassium sulfide = 110.266 g/mol K = 71% 2. Pb(ClO4)2 (% ClO4) Lead (II) perchlorate = 406.09 g/mol ClO4 = 48.98% 3. KH2PO4 (%O) Potassium dihydrogen phosphate = 136.0798 g/mol O = 47% 4. Ca3(PO4)2 (%Ca) Calcium phosphate = 310.172 g/mol Ca = 38.8% 5. NiCl2 (%Ni) Nickel (II) chloride = 129.59 g/mol Ni = 45.3% 6. Fe(NO3)3 (%NO3) Iron (III) nitrate = 241.862 g/mol NO3 = 76.9% 7. CoCl2●6H2O (%H2O) Cobalt (II) chloride hexahydrate = 237.9188 g/mol H2O = 45.4% 8. CuCl2●2H2O (%H2O) Copper (II) chloride dihydrate = 170.4796 g/mol H2O = 21.2% 9. K2SO4●10H2O (%H2O) Potassium sulfate decahydrate = 354.41 g/mol H2O = 50.8% 10. Cd(NO3)2●4H2O (%H2O) Cadmium nitrate tetrahydrate = 308.4772 g/mol H2O = 23.4%