Chapter 11 Exam Review
advertisement
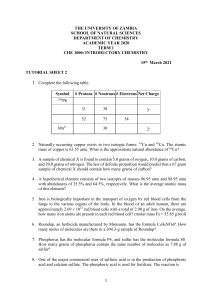
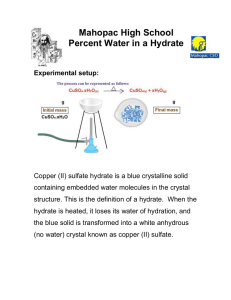
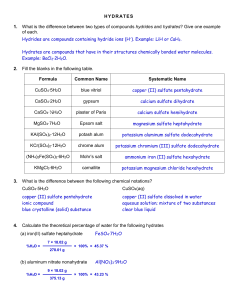
Chapter 11 Review All problem solving Two periods Calculators and periodic tables 20 questions (5 points each) Mole conversions (8) Percent composition (4) Empirical and molecular formulas (4) Hydrates (4) 1. How many molecules of CO2 are in 5.0 mol? 2. How many mol in 6.10 g of nitric acid? 3. How many atoms in 2.03 g of Cu? 4. How many grams in 4.00 x 10 26 molecules of sodium sulfide? 1. Find percent composition of oxygen in sulfurous acid. 2. Find percent composition of phosphorus in zinc phosphite. Find for a chemical that is 37.51% C, 4.20 % H, and 58.29% O. Its molecular weight is 768.56 g/mol. Find for a chemical that is 52.11% C, 13.14% H, and 34.75% O. Molecular weight is 230.39 g/mol. A hydrate of Na2SO4 is found to have a mass of 63.5 grams. After the water is driven off, the anhydrous weighs 45.99 g. Find the formula and name of the hydrate. A 20.g sample of a hydrate of nickel sulfate (NiSO4) lost 9.63g of water when heated. Determine the hydrate’s formula.