Preparation of Alkynes by Double Elimination
advertisement

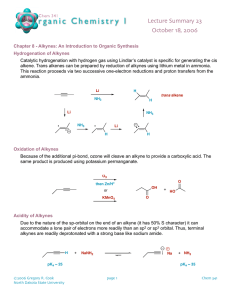
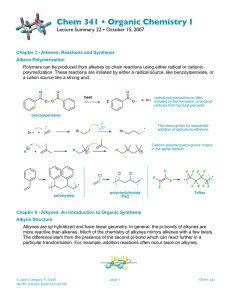
In the case of a terminal alkyne, three equivalents of base are required due to the immediate deprotonation of each alkyne formed by an equivalent of base. A synthetic sequence called halogenation-double dehydrohalogenation can be used to convert alkenes into the corresponding alkynes. Haloalkenes are intermediates in alkyne synthesis by elimination. An intermediate product in the dehydrohalogenation of a dihaloalkane is a haloalkene or alkenyl halide. With diastereomerically pure vicinal dihaloalkones, a single haloalkene product is formed due to the anti elimination reaction mechanism. 13-5 Preparation of Alkynes from Alkynyl Anions Terminal alkynyl anions will react with alkylating agents such as primary haloalkanes, oxacyclopropanes, and aldehydes or ketones. Unlike ordinary alkyl organometallic compounds, the reaction alkynyl anions with primary haloalkanes results in C-C bond formation. Reaction with secondary and tertiary halides leads to E2 products. Other reactions of alkynyl anions: 13-6 Reduction of Alkynes: The Relative Reactivity of the Two π Bonds Alkynes can undergo addition reactions, such as hydrogenation and electrophilic attack. Cis alkenes can be synthesized by catalytic hydrogenation. Catalytic hydrogenation of alkynes using hydrogen and a platinum or palladium on charcoal catalyst results in complete saturation. Cis alkenes – Use Lindlar’s Catalyst. Only reduces to Alkene (no further) Sequential one-electron reductions of alkynes produce trans alkenes. Reduction of alkynes using metallic sodium dissolved in liquid ammonia (dissolving-metal reduction) produces trans alkenes. The second electron transfer takes place faster than any cis/trans equilibrium of the alkenyl radical. The final alkene is stable to further reduction by this reagent. 13-7 Electrophilic Addition Reactions of Alkynes Addition of hydrogen halides forms haloalkenes and geminal dihaloalkanes. In an analogous reaction with alkenes, hydrogen halides add across alkyne triple bonds. The stereochemistry of this reaction is typically anti, particularly when excess halide ion is used. A second molecule of hydrogen halide may also add, following Markovnikov’s rule, producing a geminal dihaloalkane. Terminal alkynes also react with hydrogen halide, again following Markovnikov’s rule, although it is difficult to limit the reaction to a single molecule of hydrogen halide. Halogenation also takes place once or twice. Halogenation of alkynes proceeds through an isolatable intermediate vicinal dihaloalkene, to the tetrahaloalkane. The two additions are anti.