Solution Concentration & Dilution Worksheet
advertisement

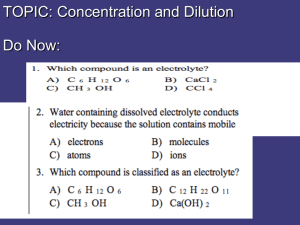
Solutions Worksheet Objectives: • Define solution concentrations • Calculate solution concentrations • Calculate solute contained in solution volumes • Perform dilution calculations 1. Define the solution concentrations: % by mass Molarity 2. Calculate the solution concentrations • What is the % by mass of this solution: 25.0g MgCl2 is added to 125.0g of water. • 0.126 moles of a substance is added to 205.0 ml. What is the molarity of the solution? • 57.65 g MgCl2 is added to water to make a solution with the final volume of 250.0 mL. What is the molarity of this solution? • 25.0 ml Alcohol is dissolved in sufficient water to give a total volume of 250.0 mL. What is the percent by volume concentration of the alcohol? 3. Solve the problems • How many grams of solute are in a 75.0ml aliquot of a 10.0% NH4Cl solution? • How many grams of solute are in a 75.0ml aliquot of a 1.25M NH4Cl solution? 4. Dilution calculations • 175.0 ml of a 2.00M NaCl stock solution and dilute it to a final volume of 1.00 L. What is the molarity of the final solution? • How would you make 200.0 ml of a 0.250M solution from a 3.25 M concentrated stock solution? • % = mass solute * 100 mass solution Answers 1. 2. 3. 4. • M = Moles solute / Liters solution Calculations • 16.7 % • 0.615M • 2.42 M • 10.0% Problems • 7.5 g • 5.01 g Dilution • 0.350 M • 15.4 ml Take 15.4 ml of stock and dilute it up to 200.0 ml with water ©2009 Dr S. Buktenica