march 29 2010 gall science ppt
advertisement

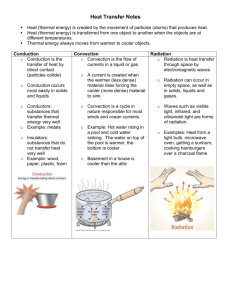
Scientific Method And much more! Mrs. Gall, March 29, 2010 Weston Preparatory Academy The Scientific Method • Link the process of a police investigation of a car accident with the stages of the scientific method. • • • • Observation/collection Hypothesis Experiments/tests Interpret/revise hypothesis • Conclusion LAB REPORT COMPONENTS • • • • • • • PURPOSE HYPOTHESIS MATERIALS PROCEDURE DATA: TABLE FORMAT AND BAR GRAPH CONCLUSION ANALYSIS • Observation/collection • The investigator examines the crime scene and fills out a report. • • • • Observation/collection Hypothesis Experiments/tests Interpret/revise hypothesis • Conclusion • Hypothesis • The investigator imagines several likely scenarios that may have led to to the accident. Maybe the driver was intoxicated, fell asleep or was speeding; maybe mechanical failure or bad weather conditions. • • • • Observation/collection Hypothesis Experiments/tests Interpret/revise hypothesis • Conclusion • Experiments/tests • The investigator might order a blood alcohol level test; check the car parts, test drive the car in different weather conditions to replicate the skid marks left by the car. • • • • Observation/collection Hypothesis Experiments/tests Interpret/revise hypothesis • Conclusion • Interpret/revise hypothesis • The investigator must reexamine evidence and possibly revise his hypothesis. The evidence may be inconclusive. • • • • Observation/collection Hypothesis Experiments/tests Interpret/revise hypothesis • Conclusion • The investigator goes to court, reexamines the evidence, and defends his theory of how the accident occurred. • Conclusion The Scientific Method • • • • Observation/collection Hypothesis Experiments/tests Interpret/revise hypothesis • Conclusion Monday, March 29, 2010 1. CHEMISTRY. Warmup: Survey: How many of the assigned conversions did you attempt? How many did you complete with confidence? 2. Scientific Method Review. 3. Lab Assignment: Compare survey results to actual performance on this weekend’s mole conversion problems. Groups formulate a hypothesis, graph data, etc. 4. Homework: Complete Conclusion and Analysis; plan to present completed lab in class tomorrow. All teams will discuss lab and quality; all will include commentary on our discussions in the final draft analysis due Weds. Monday, March 29, 2010 1. PHYSICAL SCIENCE . Warmup: In your own words, explain how heat is transferred in each of the three methods (convection, conduction, radiation) from a fireplace. Page 481 2. Notes: convection, conduction, radiation 3. Homework: Create a lab: demonstration of convection, conduction, or radiation for the classroom. Groups of 2 – 3 students (assigned). ROUGH DRAFT IS DUE TOMORROW, PLAN TO PRESENT YOUR PROGRESS IN CLASS. CONDUCTION • A thermal infrared image of a coffee cup filled with a hot liquid. Notice the rings of color showing heat traveling from the hot liquid through the metal cup. You can see this in the metal spoon as well. This is a good example of conduction. CONDUCTION • • • Conduction occurs when two object at different temperatures are in contact with each other. Heat flows from the warmer to the cooler object until they are both at the same temperature. You experience heat transfer by conduction whenever you touch something that is hotter or colder than your skin e.g. when you wash your hands in warm or cold water. CONDUCTION • • • Conduction is the movement of heat through a substance by the collision of molecules. At the place where the two objects touch, the faster-moving molecules of the warmer object collide with the slower moving molecules of the cooler object. As they collide, the faster molecules give up some of their energy to the slower molecules. The slower molecules gain more thermal energy and collide with other molecules in the cooler object. This process continues until heat energy from the warmer object spreads throughout the cooler object. Some substances conduct heat more easily than others. Solids are better conductor than liquids and liquids are better conductor than gases. Metals are very good conductors of heat, while air is very poor conductor of heat. CONVECTION • This thermal infrared image shows hot oil boiling in a pan. The oil is transferring heat out of the pan by convection. Notice the hot (yellow) centers of rising hot oil and the cooler outlines of the sinking oil. • Image courtesy of K.-P. Möllmann and M. Vollmer, University of Applied Sciences Brandenburg/Germany. CONVECTION • In liquids and gases, convection is usually the most efficient way to transfer heat. • Convection occurs when warmer areas of a liquid or gas rise to cooler areas in the liquid or gas. • As this happens, cooler liquid or gas takes the place of the warmer areas which have risen higher. • This cycle results in a continuous circulation pattern and heat is transferred to cooler areas. CONVECTION • You see convection when you boil water in a pan. • The bubbles of water that rise are the hotter parts of the water rising to the cooler area of water at the top of the pan. • You have probably heard the expression "Hot air rises and cool air falls to take its place" – this is a description of convection in our atmosphere. • Heat energy is transferred by the circulation of the air. RADIATION • A thermal infrared image of the center of our galaxy. This heat from numerous stars and interstellar clouds traveled about 24,000 light years (about 150,000,000,000,000,000 miles!) through space by radiation to reach our infrared telescopes RADIATION • • • • • • Radiation is a method of heat transfer that does not rely upon any contact between the heat source and the heated object. For example, we feel heat from the sun even though we are not touching it. Heat can be transmitted though empty space by thermal radiation. Thermal radiation (often called infrared radiation) is a type of electromagnetic radiation (or light). Radiation is a form of energy transport consisting of electromagnetic waves traveling at the speed of light. No mass is exchanged and no medium is required. HEAT TRANSFER • • • Heat can be transferred from one place to another by three methods: conduction in solids, convection of fluids (liquids or gases), and radiation through anything that will allow radiation to pass. The method used to transfer heat is usually the one that is the most efficient. If there is a temperature difference in a system, heat will always move from higher to lower temperatures. YOUR HOMEWORK: CREATE A CLASSROOM ACTIVITY • • • • • • DUE TOMORROW BRING MATERIALS BRING A DETAILED PROCEDURE IDENTIFY THE ONE CONCEPT THE ACTIVITY WILL TEACH: CONDUCTION, CONVECTION, OR RADIATION HAVE TWO QUESTIONS TO TEST THE STUDENT’S UNDERSTANDING YOU WILL GIVE YOUR LAB AND MATERIALS TO ANOTHER GROUP. THEY WILL DO YOUR ACTIVITY AND EXPLAIN WHAT THEY LEARNED BY ANSWERING THE QUESTIONS. SELECT GROUPS MAY PRESENT THEIR ACTIVITY TO THE CLASS.

![Applied Heat Transfer [Opens in New Window]](http://s3.studylib.net/store/data/008526779_1-b12564ed87263f3384d65f395321d919-300x300.png)

