Heat Transfer Notes
advertisement
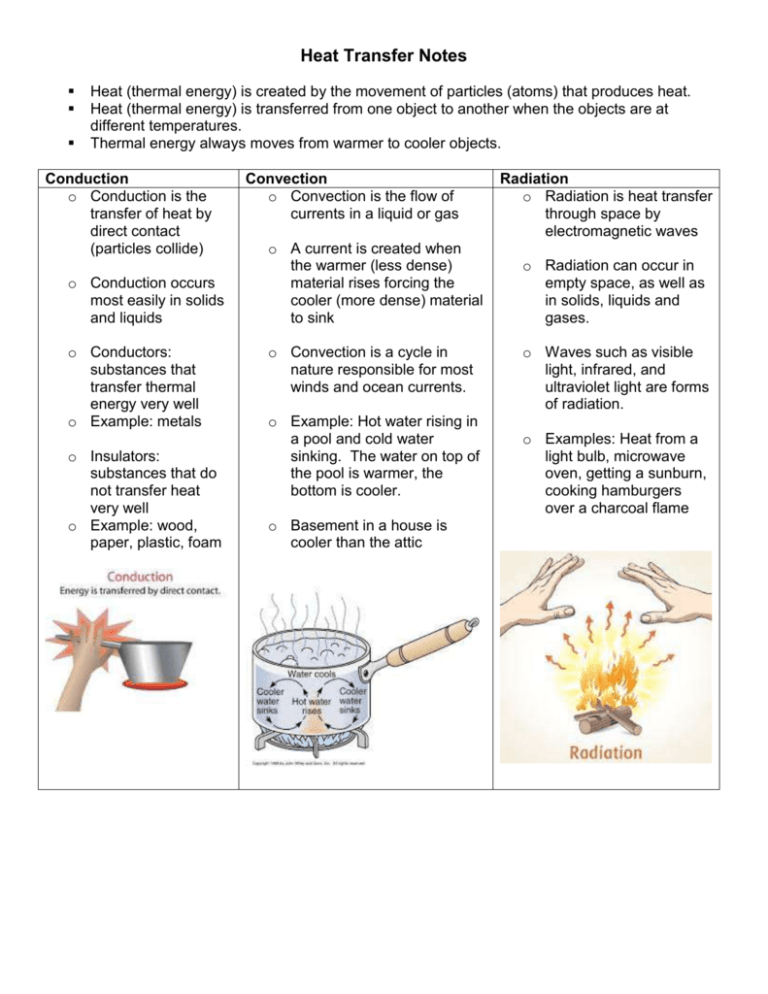
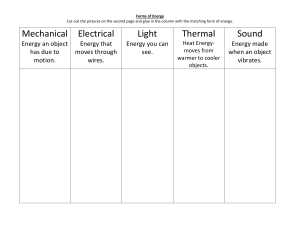
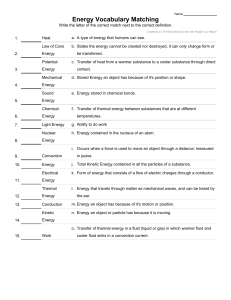
Heat Transfer Notes Heat (thermal energy) is created by the movement of particles (atoms) that produces heat. Heat (thermal energy) is transferred from one object to another when the objects are at different temperatures. Thermal energy always moves from warmer to cooler objects. Conduction o Conduction is the transfer of heat by direct contact (particles collide) o Conduction occurs most easily in solids and liquids o Conductors: substances that transfer thermal energy very well o Example: metals o Insulators: substances that do not transfer heat very well o Example: wood, paper, plastic, foam Convection o Convection is the flow of currents in a liquid or gas o A current is created when the warmer (less dense) material rises forcing the cooler (more dense) material to sink o Convection is a cycle in nature responsible for most winds and ocean currents. o Example: Hot water rising in a pool and cold water sinking. The water on top of the pool is warmer, the bottom is cooler. o Basement in a house is cooler than the attic Radiation o Radiation is heat transfer through space by electromagnetic waves o Radiation can occur in empty space, as well as in solids, liquids and gases. o Waves such as visible light, infrared, and ultraviolet light are forms of radiation. o Examples: Heat from a light bulb, microwave oven, getting a sunburn, cooking hamburgers over a charcoal flame