Biochemistry notes (updated 10/26)
advertisement

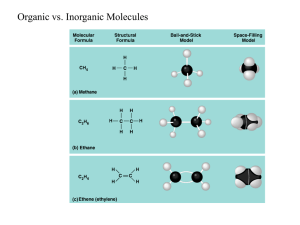
Are you what you eat? 1. The important Characteristics of Carbon Forms 4 covalent bonds Forms double and triple bonds Forms long chains and rings Can bind with many other elements Even electron distribution (nonpolar molecules) 2. Macromolecules, Monomers and Polymers (Hint: think of the meaning of the prefixes) What do these words mean? Polygons Polyester Polygamy 2. Macromolecules, Monomers and Polymers Polymer – Smaller organic molecules join into long chains. Monomer – the individual unit that builds up polymers Macromolecules – Very large molecules 3. Dehydration synthesis and Hydrolysis These two terms refer to the processes that forms monomers and polymers: Dehydration synthesis – A reaction that removes molecules of water to form polymers from monomers Hydrolysis – The reaction that adds water to polymers to separate them to their individual monomers. (http://nhscience.lonestar.edu/biol/dehydrat/dehydrat.html or http://www.youtube.com/watch?v=UyDnnD3fMaU ) Isomers Molecules that have the same formula, but different structures. Examples: Glucose and Fructose 4. What are the big four? Three out of the 4 types of biochemical macromolecules can be found on food nutrition labels… Look at the label to the left. 3 of the 4 macromolecules can be found in foods. 1____________________ (0 grams in this product) (13 grams in this product) 2____________________ (9 grams in this product) 3____________________ 4. What are the big four? Fats (we call them lipids) Carbohydrates Proteins Nucleic acids (DNA and RNA) When studying these biochemical molecules, we are interested in finding out….. what they do for living things. what they generally look like. what their monomers are. and how they may help the body gain energy to sustain life. SO, LETS GET STARTED! Great website for reference… http://biomodel.uah.es/en/model3/index.htm 5. Carbohydrates Molecules that form from atoms in C1:H2:O1 ratio Monomers: Monosaccharides (simple sugars) Monosaccharides are usually sweet, white powdery substances (such as fructose, glucose) that form rings of carbon atoms. Monosaccharides in general serve as direct, quick sources of energy for living organisms during cellular respiration, they are building blocks of many polymers Important monosaccharides: Glucose Fructose Disaccharides – two monosaccharide molecules combine by dehydration synthesis to form disaccharides Important disaccharides: Lactose – found in milk sugar Sucrose – table sugar Polysaccharides – many (tens to hundreds) units of monosaccharides combine by dehydration synthesis Polysaccharides also separate to monosaccharides by hydrolysis while taking in water. Important polysaccharides: Starch – made up of many glucose units, it is an important storage polysaccharide that is found in plant roots and other tissues. It stores monosaccharides that can be broken down later to release useful energy during cellular respiration – ONLY IN PLANTS Glycogen – also made up of many glucose units, it is an important storage polysaccharide in the liver and animal muscles. It can also be broken down to monomers to release energy during cellular respiration. ONLY IN ANIMALS Cellulose – also made up of many glucose units. However, in this case the molecule is not easily broken down to its monomers. It is important for providing a rigid structure in plant cell walls. Chitin – made up of some nitrogen containing monosaccharides. It is an important polysaccharide that provide the solid structure of arthropods and fungi. 6. Lipids a diverse group of molecules that are nonpolar and generally do not dissolve in water They mostly contain carbon, hydrogen, very few oxygen atoms, but some also have phosphorous. There are three distinct groups of lipids: Simple lipids Phospholipids Sterols 6A. Simple Lipids Very large molecules that form from 2 different kinds of monomers by dehydration synthesis: 3 Fatty acids – are long chains of carbon with oxygen at the end (can be saturated and unsaturated) 1 Glycerol – smaller 3-carbon compound. Simple lipids are important as storage materials in all living things. They can store twice as many calories as polysaccharides can. Oils (mostly from plants) contain more unsaturated fatty acids, while fats (animals) contain more saturated fatty acids. Simple lipids also dissolve vitamins http://biomodel.uah.es/en/model3/index.htm 6B. Phospholipids Phospholipids – phosphate containing lipids. Their monomers: 1 glycerol + 2 fatty acids (saturated or unsaturated) + phosphate. These monomers combine by dehydration synthesis Phospholipids have both polar and nonpolar sections. As a result, they are able to dissolve in both type of solvents as well. They are important for living things because they form the borders of all cells (cell membranes) and also participate in forming many cell organelles. 6C. STEROLS Sterols are a highly nonpolar (hydrophobic) group of molecules. They occur naturally in plants, animals, and fungi, with the most familiar type of animal sterol being cholesterol. Cholesterol is vital to cellular function, and a precursor to fat-soluble vitamins and steroid hormones. 3-six sided rings and one 5-sided ring + alcohol 7. Proteins Protein- Polymer constructed from amino acid monomers. Only 20 amino acids, but make 1,000s of proteins Some are 100 a.a. in length; some are thousands 3-D Protein 7A. Protein Functions Each of our 1,000s of proteins has a unique 3-D shape that corresponds to a specific function: Defensive proteins Antibodies in your immune system Signal proteins Hormones and other messengers Hemoglobin Delivers 02 to working muscles Transport proteins Move sugar molecules into cells for energy (insulin) Storage proteins Ovalbumin (found in egg white) used as a source of amino acid for developing embryos Most important roles is as enzymes Chemical catalysts that speed and regulate virtually all chemical reactions in cells Example, lactase 7B. Amino Acid structure Proteins diversity is based on differing arrangements of 20 amino acids. Amino acids all have an amino group and a carboxyl group. R group is the variable part of the amino acid; determine the specific properties of the 20 amino acids. Two main types: Hydrophobic Example: Leucine R group is nonpolar and hydrophobic Hydrophilic Polar and charged a.a.’s help proteins dissolve in aqueous solutions inside cells. Example: Serine R group is a hydroxl group 7C. Amino Acid Dehydration Cells join amino acids together in a dehydration reaction: Links the carboxyl group of one amino acid to the amino group of the next amino acid as a water molecule is removed. Form a covalent linkage called a peptide bond making a polypeptide. 7D. Protein Structure Primary Structure Unique sequence of amino acids For any protein to perform its specific function, it must have the correct collection of amino acids arranged in a precise order. Example: a single amino acid change in hemoglobin causes sickle-cell disease Determined by inherited genetic information. 7D. Protein Structure Secondary Structure Parts of the polypeptide coil or fold into local patterns. Patterns are maintained by regularly spaced hydrogen bonds between the hydrogens of the amino group and the oxygen of the carboxyl groups. Coiling results in an alpha helix. Many fibrous proteins have the alpha structure over most of their length Example: structural protein of hair Folding leads to a pleated sheet. Make up the core of many globular proteins Dominate some fibrous proteins, including the silk proteins of a spider’s web 7D. Protein Structure 7D. Protein Structure Tertiary Structure Overall, three-dimensional shape of a polypeptide. Roughly describe as either globular or fibrous Generally results from interactions among the R groups of amino acids making up the polypeptide. 7D. Protein Structure Quaternary Structure Results from association of subunits between two or more polypeptide chains. Does not form in every protein. Example, Hemoglobin 8. Nucleic Acids DNA and RNA Deoxyribonucleic Acid (DNA) Monomers made up of nucleotides: Nucleotides consist of: A five carbon sugar, deoxyribose Phosphate group Nitrogenous base (Adenine, Guanine, Cytosine, Thymine) Double helix consists of: Sugar-phosphate backbone held by covalent bonds Nitrogen bases are hydrogen bonded together; A pairs with T and C pairs with G 8A. Nucleotides of DNA 8B. DNA Genetic material that organisms inherit from their parents. Genes Specific stretches of DNA that program amino acid sequences of proteins. 8C. RNA Ribonucleic Acid (RNA) Intermediary for making proteins Single-stranded Also made up of monomers of nucleotides Nucleotide of RNA: Sugar is ribose (not deoxyribose) Phosphate group Nitrogen bases (Adenine, Uracil (instead of Thymine, Guanine, and Cytosine) 9. Enzymes (First half of chapter 5) Before we can understand how these important proteins function, we are going to look at: Types of Energy Chemical Reactions ATP 9A. Types of Energy Energy: The capacity to perform work Potential energy A form of potential energy is chemical energy (energy of molecules) Kinetic energy A form of kinetic energy is heat 9A. Types of Energy Thermodynamics: the study of energy transformations that occur in a collection of matter. 1.1st Law of Thermodynamics Law of energy conservation energy cannot be created nor destroyed; energy can only be transferred and transformed By converting sunlight to chemical energy, plants are acting as an energy “transformer,” not an energy producer. 9A. Types of Energy 2.2nd Law of Thermodynamics Energy conversions reduce the order of the universe and increase its entropy (the amount of disorder in a system). During every energy transfer or transformation, some energy becomes unusable. In most energy transformations, some energy is converted to heat, the energy associated with random molecular motion. When muscle cells convert the chemical energy of food molecules to kinetic energy, more than half of the energy is lost as heat. Released during sweating. 9B. Chemical Reactions Chemical reactions can store or release chemical energy. endergonic – a reaction where energy is taken in by the reactants to form the products (like dehydration synthesis or photosynthesis) exergonic – a reaction where energy is released by the reactants to form the products (like cellular respiration) Frequently, exergonic reactions fuel endergonic reactions – energy coupling 9B. Chemical Reactions 9C. ATP (adenosine triphosphate) ATP: A modified nucleotide molecule that powers all cellular work directly. Its structure: adenine, ribose and three phosphates are combined by dehydration synthesis 9C. ATP Phosphorylation ATP molecules release phosphate groups to various other molecules. These molecules take in the phosphate by phosphorylation and get excess energy to perform various processes. When ATP releases a phosphate + energy it produces ADP (adenosine diphosphate) ADP can turn back to ATP by taking in a phosphate and energy by phosphorylation 9C. ATP http://www.biologyinmotion.com/atp/index.html http://student.ccbcmd.edu/biotutorials/energy/atpan.html 9C. ATP The energy from ATP can be used for the following processes: Chemical work (forming products from reactants) Mechanical work (contracting muscle) Transport work (moving substances into or out of the cell) 10. Enzymes Enzymes are proteins that act as biological catalysts in living organisms. They speed up chemical reactions by lowering the activation energy of the reaction. http://www.stolaf.edu/people/ giannini/flashanimat/enzymes /transition%20state.swf 10A. Enzyme Specificity Enzymes have a specific section called the active site that is able to bind with the reactants (substrates) of a chemical reaction Once the substrates bind to the active site, the active site changes shape and pulls the reactants together. As a result, the reaction occurs faster and more efficiently. The model that describes that enzymes change shape when bind with the substrate is called the induced fit model 10B. Induced Fit Model Animations: http://highered.mcgrawhill.com/sites/0072495855/student_view0/c hapter2/animation__how_enzymes_work.ht ml http://www.lpscience.fatcow.com/jwanamak er/animations/Enzyme%20activity.html http://www.northland.cc.mn.us/biology/biolo gy1111/animations/enzyme.swf 10C. Enzyme Characteristics Three important special characteristics of enzymes: They are specific They are efficient They are sensitive 10D. Cofactors and Inhibitors Cofactors: Many enzyme do not function without an additional group attached to them. This additional group is called a cofactor. Inhibitors: Some substances can stop enzymes from functioning by attaching themselves to the active site of the enzyme. These are called inhibitors. Many inhibitors are used as poisons or drugs.