© SSER Ltd.
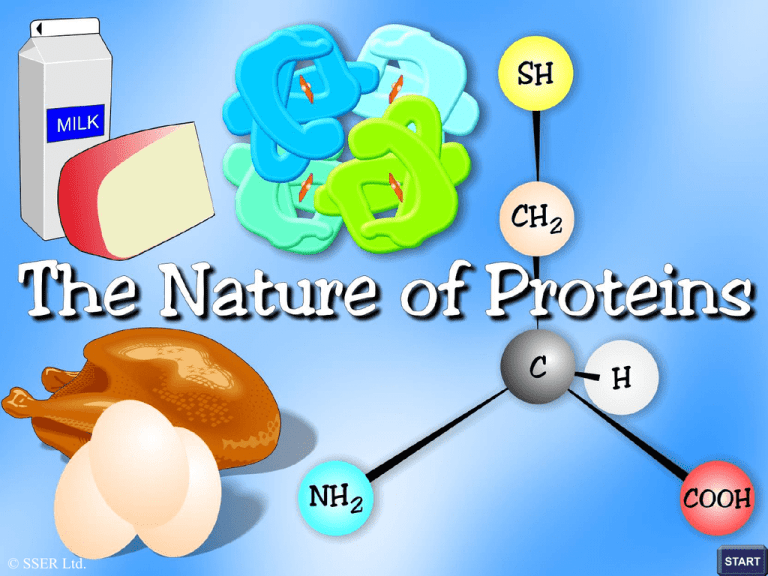
The Nature of Proteins
The significance of proteins cannot be over-emphasised, since
they are intimately connected with all phases of the chemical
and physical activities of the living cell
Proteins function as enzymes, hormones and oxygen transporters
and they form the bulk of skin, hair, feathers, nails and cartilage
Human
hair
Feather
Proteins are huge three-dimensional molecules whose building
blocks or monomers are the variety of different amino acids
found in nature
Amino Acid Structure
Variable group
amino
group
C
C
carboxylic
acid group
Formation of a Dipeptide
C
TWO
AMINO
ACIDS
C
C
C
C
O
N
D
E
N
S
A
T
I
O
N
-H
O
2
C
C
C
p
e
p
t
i
d
e
b
o
n
d
C
D
I
P
E
P
T
I
D
E
The Variety of Amino Acids
Individual amino acids display a
tetrahedral shape due to the angles
of the bonds between the atoms
There are twenty different naturally
occurring amino acids that differ from
one another by virtue of the R group
The simplest of the amino acids
possesses a hydrogen atom for its R
group. This amino acid is called
GLYCINE
The Variety of Amino Acids
R GROUP
This amino acid, known as
ALANINE, possesses a CH3 group
as its R group
This amino acid possesses
one acid and one basic group.
Overall, this amino acid is
a NEUTRAL MOLECULE
The amino group
The carboxylic acid
is a basic group group is an acidic group
The Variety of Amino Acids
SH
R GROUP
This amino acid has sulphur
in its R group. This amino acid
is called CYSTEINE
C
There are only TWO sulphur-containing amino acids;
THESE ARE CYSTEINE AND METHIONINE
Proteins - Levels of Structure
Amino Acid Sequence
Peptide bond
Polypeptide chains form when amino acids bond together
in a particular sequence. THE PRIMARY STRUCTURE
of a protein is the number, type and sequence of amino acids
that make up this linear chain together with the peptide bonds
that hold them together
Different proteins have different primary structures. Different
proteins are made up of different types, numbers and sequences
of amino acids making up the primary chain
Proteins - Levels of Structure
Secondary Structure
The secondary structure of proteins is the arrangement in
space of the atoms that form the backbone or linear chain
of the protein
The amino acid chain can coil into a helix shape or form
a shape called the beta pleated sheet
The helix and beta pleated sheet shapes are secondary
structures of protein molecules
Alpha helix
Beta pleated sheet
Secondary Structure of Proteins
The Alpha Helix
HYDROGEN
BOND
The amino acid chain
coils into a right-handed
helix and hydrogen
bonds form between
oxygen and hydrogen
atoms that have been
brought into close
proximity
These hydrogen bonds
help to stabilise this
secondary structure
Secondary Structure of Proteins
The Beta-Pleated Sheet
The hydrogen bonds help to stabilise
this secondary structure
The amino acid
chain folds back
upon itself many
times forming
anti-parallel
chains. The oxygen
and hydrogen
atoms that have
been brought into
close proximity
form hydrogen
bonds
Tertiary Structure of Proteins
HAEM GROUP
MYOGLOBIN MOLECULE
All globular proteins
display tertiary structure.
Once the secondary
structures have formed,
the molecule bends and
folds into a 3-D globular
shape
Myoglobin is a globular
protein found in muscle
cells. This tertiary shape
is the highest level of
structure for this protein
and a variety of bonds
help to stabilise its
structure
Quaternary Structure of Proteins
beta chain
beta chain
Quaternary structure is a
level of structure displayed
by proteins that consist of
more than one polypeptide
chain
Haemoglobin is a protein
displaying quaternary
structure
Haemoglobin consists of four
polypeptide chains that are
held together by weak van
der Waals forces
alpha chain
alpha chain
iron-containing
haem group
The Haemoglobin Molecule
Each polypeptide chain contains
an iron containing HAEM group
that binds to molecules of oxygen
BONDS THAT STABILISE SECONDARY & TERTIARY
STRUCTURE
As the chains of amino acids bend
& fold to form secondary & tertiary
structures, various atoms are brought
into close proximity and form bonds
Hydrogen and oxygen atoms from both the
main chain and the R groups may form
hydrogen bonds
The R groups of two amino acids contain
sulphur atoms. When these atoms are in
close proximity they form DISULPHIDE
BRIDGES
Many of the carboxylic acid and amino groups
form charged groups in solution. Oppositely
charged groups form IONIC BONDS
Many hydrophobic R groups tend to cluster
towards the interior of the protein molecule
forming Hydrophobic Interactions
SUMMARY
The building blocks of proteins are monomers
called amino acids
Every amino acid possesses an amino end and a
carboxylic acid end
There are twenty different naturally occurring
amino acids
Amino acids differ by virtue of the nature of
their R groups
Amino acids bond together forming peptide
bonds
When two amino acids bond during a
condensation reaction, the resulting molecule is
a dipeptide
When many amino acids bond together, the
resulting molecule is referred to as a
polypeptide
Chains of amino acids numbering greater than
100 are generally referred to as proteins
Individual amino acids may be neutral, basic or
acidic
Two amino acids, namely cysteine and
methionine, possess sulphur atoms in their R
groups
The type, number and sequence of amino acids
forming the original linear chain of a protein is
termed the PRIMARY STRUCTURE OF A
PROTEIN
Different proteins have different primary
structures
The primary structure determines the final
shape of the protein molecule
The linear chain of amino acids making up the
primary structure of the protein bends and folds
in various ways to form the SECONDARY
STRUCTURE OF THE PROTEIN
Two main types of secondary structure are found
in proteins - the beta pleated sheet and the alpha
helix
The alpha helix forms when the linear chain coils
into a right handed helix
The beta pleated sheet forms when the linear
chain folds back on itself many times
Hydrogen bonds play a major part in stabilising
the secondary structure of proteins
Many proteins bend and fold further to form
globular TERTIARY STRUCTURES
Myoglobin is a globular protein displaying the
tertiary level of structure
Myoglobin is a protein found in muscle cells
Proteins consisting of more than one polypeptide
chain display quaternary structure
Haemoglobin is a protein consisting of more than
one polypeptide chain
Haemoglobin consists of four separate polypeptide
chains held together by weak van der Waals forces
Each polypeptide chain in haemoglobin contains a
haem group that binds to molecular oxygen
The role of haemoglobin is to transport oxygen
molecules from the lungs to the body tissues
A variety of different bonds stabilise the
secondary and tertiary structures of proteins
Hydrogen bonds form between oxygen and
hydrogen atoms within the main amino acid
chain and between the R groups
Disulphide bridges form between sulphur
atoms in the R groups of amino acids such as
cytseine
Ionic bonds form between charged amino
groups and charged carboxylic acid groups
Hydrophobic interactions occur between R
groups that have clustered towards the centre
of protein molecule due to their hydrophobic
nature
Acknowledgements
Copyright © 2003 SSER Ltd. and its licensors.
All rights reserved. All graphics are for viewing purposes only.







