Chemical Kinetics Chapter 14
advertisement

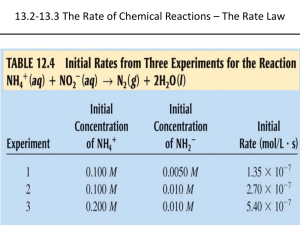
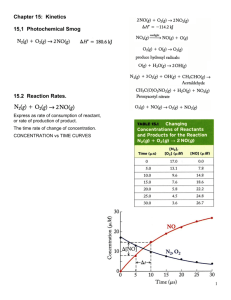
Chemical Kinetics Chapter 14 • Chemical kinetics is concerned with the speeds or rates of reaction. Section 1 • collision theory states that the greater frequency of collisions the greater the rate of reaction. 4 Factors that Affect Reaction Rates • (based on collision theory) • Physical state of the reactants. If the reaction involves a solid the reaction will have a faster rate if the solid is in powder form. ( more surface area) 4 Factors that Affect Reaction Rates • (based on collision theory) Concentrations of the reactants. The more concentrated the reactants the more often collisions occur 4 Factors that Affect Reaction Rates • (based on collision theory) Concentrations of the reactants. The more concentrated the reactants the more often collisions occur 4 Factors that Affect Reaction Rates • (based on collision theory) Temperature at which the reaction occurs. The greater the temperature the greater the kinetic energy of collisions and more often collisions occur. 4 Factors that Affect Reaction Rates • (based on collision theory) Temperature at which the reaction occurs. The greater the temperature the greater the kinetic energy of collisions and more often collisions occur. 4 Factors that Affect Reaction Rates • (based on collision theory) • Presence of a catalyst- a agent that increases reaction rate without being used or permanently changed in the process. Section 2 Reaction Rates Reaction rates- the change in concentration of reactants or products per unit time Section 2 Reaction Rates Reaction rates- the change in concentration of reactants or products per unit time Section 2 Reaction Rates The units for reaction rate is molarity per unit time (mol/L) = M. Section 2 Reaction Rates • Pg 527 figure 14.3 •A → B Section 2 Reaction Rates The reaction rate can be given as either appearance of product B or disappearance of reactant A Average rate with respect to B = Δ [B]/t Section 2 Reaction Rates * The brackets indicate concentration * Δ means change in and is always equal to final minus initial Section 2 Reaction Rates • Rates are always expressed as positive quantities. So if looking at a disappearing reactant the rate is the opposite of the change in concentration per unit time. • Average rate with respect to A = Δ [A]/t Sample exercise 14.1 Using figure 14.3 page 527 • Calculate the average rate of disappearance of A over the time interval 20s to 40s. • @ t = 20s [A] = .54M • @ t = 40s [A] = .31M Sample exercise 14.1 Using figure 14.3 page 527 • Average rate = - (.3M - .54M) = ( 40s - 20s ) 1.2 x 10-2M s Practice Exercise pg 528 Calculate the average rate of appearance of B over the time interval 0 to 40 s. @ t = 0 s [B] = 0M @ t = 40s [B] = .7 M Practice Exercise pg 528 Calculate the average rate of appearance of B over the time interval 0 to 40 s. • rate = .7M – 0M = 1.8 x 10-2 M 40s - 0s s Change of Rate with Time It is typical for reaction rates to decrease over time because concentration of reactants change over time. Page 529 figure 14.4 ( notice that the steepness of the graph decreases with time.) • instantaneous rate- rxn rate at a particular moment in the reaction. • Instantaneous rate is determined from the slope of the tangent line at the point of interest. • The instantaneous rate at t=0 is called an initial rate. Sample exercise 14.2 page 530 Looking at 600 s [.017] @ 800 s [.042] @ 400s Instantaneous rate at 600s 017 - .042 = 6.2 x 10-5 M 800s – 400s s Reaction Rates and Stoichiometry • Both examples we have done so far require that the rate of appearance of the product = the rate of disappearance of the product. The is because the stiochiometry was 1:1 When stiochiometric relationships are not one to one: 2HI → H2 + I2 Rate = - ½ Δ[HI]/t = Δ[H2]/t = Δ[I2]/t In general for the reaction ( lower case letters are coefficients) aA + bB → cC + dD Rate = -1/a Δ[A]/t =-1/b Δ[B]/t = 1/c Δ[C]/t = 1/d Δ[D]/t Sample exercise 14.3 page 531 a.)How does the rate the disappearance of O3 compare to the rate of appearance of O2 ? 2O3 → 3O2 Rate = -1/2 [O3]/t = 1/3 [O2]/t b.) – [O3] = 2/3 Δ [O2] = 4.5 10-5 mol O3 /L s • 14.5, 14.9 14.11 in class (back of chapter) 14.3 Concentration and Rate One way to study the effect of concentration is the consider how the initial rate reaction depends on initial concentration. NH4+ + NO2- → N2 + 2H2O • Table 14.2 page 532 • What happens to rate when [NO2] is kept constant and [NH4+] is doubled? Rate doubles. The same thing happens to the rate when [NH4+] is kept constant and [NO2 ] is doubled. The rate of the reaction is proportional to the [NH4+] raised to first power and to [NO2 ] raised to the first power. Rate = k [NH4+][NO2-] Rate law – an equation that shows how the rate depends on the concentration of reactants In general for: aA + bB → cC rate = k [ m A] n [B] + dD • k is the rate constant and changes w/ temp. • m and n are typically small whole numbers and are determined by how the concentration of the reactant affects rate • The exponents m and n are called reaction orders If the rate is directly proportional to the concentration of the reactant the exponent is 1. For example first order in [NH4+] and first order [NO2-]. Rate=k[NH4+][NO2-]. If the rate is directly proportional to the concentration of the reactant the exponent is 1. For example first order in [NH4+] and first order [NO2-]. Rate=k[NH4+][NO2-]. An exponent of 1 is never written. An exponent of 0 means that the concentration of that particular reactant does not influence rate. An exponent of 1 means that the if the concentration is doubled the rate is doubled. ( directly proportional) An exponent of 2 means that if the concentration is doubled the rate is quadrupled. note: often the exponents are the same as the coefficients, but not always. The exponents must be determined experimentally. It is possible to have a reaction order that is a fraction. • • • • • Sample exercise 14.4 Box 1 : Box 2 : Box 3 : ___> ____> ___ • • • • • Sample exercise 14.4 Box 1 : k (5)(5)2 = 125 k Box 2 : k (7)(3)2 = 63 k Box 3 : k (3)(7)2 = 147 k 3> 1> 2 • • • • • Practice exercise 1= 2= 3= ___=____<___ • • • • • Practice exercise 1 = k(5)(5) = 25 k 2= k (7)(3) = 21k 3 = k (3)(7) = 21k 2=3<1 Units of Rate Constants The units of the rate constant depend on the overall reaction order of the rate law Unit of rate const= units of rate/(units of conc.)overall rxn rate • 1st order • 2nd order • 3rd order = M/s = s-1 M = M/s = M-1s-1 M2 = M/s = M-2s-1 M3 Sample exercise 14.5 a.) b.) c.) Sample exercise 14.5 a.) 1st order b.) 3/2 order c.) 2nd order Practice exercise 535 Using Initial Rate to Determine Rate Laws • The rate law for any experiment must be determined experimentally. It is found by observing the effect of changing initial conc of reactants. Most reactants have exponents of 0, 1, or 2. The rate of a reaction depends on concentration but rate constant does not. Rate constant is affected by temperature and presence of a catalyst. Sample exercise 14.6 Pge 536 Rate law = k [A]m[B]n Rate law = k = __________= rate = Sample exercise 14.6 Pge 536 Rate law = k [A]m[B]n Rate law = k [A]2[B]0 = k [A]2 k = 4 X 10-5 = 4 X 10-3 M-1s-1 [.1M]2 rate = (4 X 10-3)(.05 M) = 1X 10 -5 M/s Practice 536 rate = k= Rate = Practice 536 rate = k [NO]2[H2] k = 1.23 x 10-3 M/s = 1.23 M-2s-1 (.1M)2 (.1M) Rate = (1.23 M-2s-1)( .05M)2(.15M) = 4.61 x 10-4 M/s Page 565 14.13, 15, 17, 21 and 23 Example 14.6 Rate = k [A]m[B]n m=____ b/c rate increases by ____ when [ ] increases by 2 n= ____ b/c rate doesn’t change when conc. is doubled Example 14.6 Rate = k [A]m[B]n m=2 b/c rate increases by 4 when [ ] increases by 2 n= 0 b/c rate doesn’t change when conc. is doubled rate = k= c.) rate = k [A]2 k = rate/[A]2 = 4 x 10-5M/s = 4 x 10-3M-1s-1 (.1M)2 c.) (4 x 10-3)(.05M)2 = 1 x 10 -5 M/s Practice pg 537 Rate = k= Practice pg 537 Rate = k[NO]2[H2] k= rate/[NO]2[H2] = 1.23 x 10-3 M/s = 1.2 M-2s-1 (.1M)2(.1M) 13.) a.) b.) c.) k = 13.) a.) increase by a factor of 4 No the rate constant remains unchanged. When [A] increases the rate is increased. b.)second order in A; first order in B; third order overall. c.) k = M/s = M-2s-1 (M2)(M) 15.) a.) b.) c.) 15.) a.) Rate = 4.82 x 10-3 s-1[N2O5] b.) rate = 4.82 x 10-3s-1(.024M)= 1.15 x 10-4M/s c.) rate is doubled to 2.3 x 10 -4 M/s 17. ) Rate = a.) b.) c.) 17. ) Rate = k [CH3Br][OH-] .0432 m/s =k ( 5 x 10-3)(.05) a.) 1.728 x 102 M-1s-1 =k b.) M-1s-1 c.) Rate would be tripled 21. a.) rate = k[OCl-][I-] 1.36 x 104 M/s (1.5 x 10-3M)( 1.5 x10-3M) b.) 6.0 x 109 M-1s-1 = k rate = ( 6.0 x 109 M-1s-1) ( 1.0 x 10-3M)(5x10-4M) = 3 x 103M/s 21. a.) rate = b.) rate = 23. a. rate = k[NO2]2[O2] b.) - k= M/s = M-2s-1 M2 M c.) - 1.4 x 10-2 = 7.05 x 10 3 M-2s-1 (0.126)2(.0250) 1.13 x10-1 = 7.1 x 10 3 M-2s-1 (.0252)2(.0250) 5.64 x 10-2 (.0252)2(.0125) = 7.1 x 10 3 M-2s-1 Section 4 The Change of Concentration with Time In this section we will use equations that will tell us the concentrations of reactants and products at any given time. First Order Reactions Rate depends on the concentration of a single reactant raised to the first power. Rate = - Δ[A]/t= k [A] using integration we can get an equation that relates the initial concentration of A at [A]◦ to the concentration at any other time [A]t ln[A]t – ln [A]◦ = -kt or ln [A]t = -kt [A]◦ or ln[A]t = -kt + ln [A]◦ ( slope intercept form) These equations can be used to find [A]t , [A]o , t or k if any three of those are known. Example 14.7 b/c ln[A]t = -kt + ln [A]◦ is in slope intercept form, (y=mx +b) the graph of ln[A]t vs. time will give a straight line with slope –k and y intercept ln[A]o If a reaction is first order this graph will give a straight line. some example problems will use pressure as a unit of concentration for a gas. Pressure is directly proportional to the number of moles per unit volume and can be used for concentration. Second Order Reactions A reaction whose rate depends on the reactant concentrations raised to the second power or on the concentration of two reactants to the first power. For a rxn that is second order in just one reactant: Rate = - Δ[A]/t= k [A]2 Rate = - Δ[A]/t= k [A]2 With calculus the rate law can be written: 1 = kt + 1 [A]t [A]o This above equation can be used to find [A]t, [A]o , t or k for second order reactions in one reactant. The graph of 1/[A]t vs t will yield a straight line with slope k and y intercept 1/[A]o One way to distinguish first order reactions from second order reactions is to plot ln[A]t vs. time and 1/[A]t whichever plot gives a straight line tells you if the rxn is first order or second order in one reactant. These are the two of the most common types. • [A]t – first order • 1/[A]t- second order example 14.8 Half-Life The half life t1/2 is the time required for the concentration of a reactant to drop to one half of its initial value. For a first order rxn: [A]t1/2 = ½ [A]o ln ½ [A]o = -kt1/2 [A]o ln ½ = -kt1/2 ln ½ = t1/2 -k .693/k = t1/2 The half life for a first order rxn is independent of initial conc. of reactant. In a first order rxn. The conc. of the reactant decreases by ½ in each of a series of regularly spaced time intervals. The half life for a second order and other reactions depends on the reactant concentrations and therefore changes as the reaction progresses. T1/2 = 1/k[A]o • Example 14.9 Section 5: Temperature and Rate • The rates of most chemical reactions increases as temp increases • As temp increases, the rate constant increases Collision Model Molecules must collide to react. The larger the number of collisions per second, the greater the reaction rate. As temperature increases, the molecules move faster and collide more often and with larger KE Only a tiny fraction of collisions lead to rxn •molecules must be oriented correctly for a collision to result in a rxn. •Molecules must have enough activiation energy (Ea) activation energy- in order to react the KE of the molecules must be large enough to stretch, bend, and break bonds. So that the chem. rxn. can occur. Activation energy can be considered a barrier that represents the energy necessary to force a molecule through a relatively unstable intermediate stage to the final product. Ea is the difference in energy between the starting molecule and the highest energy along the reaction pathway. • The most unstable complex is the activated complex or transition state. • Generally the lower the activation energy the faster the reaction. As the temp increases a greater fraction of molecules will have the KE necessary to overcome Ea which leads to a larger reaction rate. • The fraction of molecules with enough energy to overcome Ea is given by : • f=e-Ea/RT • • f – fraction of molecules with sufficient energy to overcome Ea • T- temperature in Kelvin • R – gas constant – 8.314 J/mol • K • For a temp of 300 K , Ea of 100,000 J/mol ( a typical value) f= 3.8 x 10-18 moral of the story “ at any given time the fraction of molecules present with sufficient energy is extremely small Arrhenius Equation • The increase in the rate with increasing temp is nonlinear. • Rxn rate is dependent on 3 factors • fraction of molecules with energy equal to Ea or more • # of collisions per second • fraction of collisions with appropriate orientation • • • • • • • • Arrhenius Equation k= Ae-Ea/RT k – rate constant Ea – activation energy R is gas constant ( 8.314 J/mol•K) T in Kelvin A is the frequency factor The frequency factor is constant as temperature is varied • Rxn rates decrease as Ea increases Exercise 4.10 Determining the Activation Energy • Taking the natural log of both sides of the Arrhenius equation gives • ln k = - Ea/ RT + ln A The graph of ln k vs. 1/T will give a straight line with slope -Ea/R and y intercept ln A Or non-graphically If you know the rate constants at two temps. Ln k1/k2 = Ea/R ( 1/T2 - 1/T1) Or Ea = -( (ln k1 - lnk2)/ (1/T1- 1/T2))R Exercise 14.11 T1 = 230 C ( 503K) and k1= 6.3 x 10-4 T2 = 189.7C ( 462.7 K) and k2 = 2.52 x 10-5 Ea = - ((ln _______– ln ________)/ 1/_____ 1/_______)) 8.314 = ___________J/mol • • • • • Exercise 14.11 T1 = 230 C ( 503K) and k1= 6.3 x 10-4 T2 = 189.7C ( 462.7 K) and k2 = 2.52 x 10-5 Ea = - ((ln 6.3 x 10-4 – ln 2.52 x 10-5)/ 1/503 1/462.7)) 8.314 • = 1.6 x 105 J/mol • Section 6 Reaction Mechanisms The process by which a reaction occurs is called the reaction mechanism At the most detailed level a rxn mechanism will describe exactly the order in which bonds are broken and reformed. Elementary Steps When a reaction occurs in a single step, the process or steps are called elementary steps. The number of molecules that participate as reactants in an elementary step defines the molecularity of the step If a single molecule is involved it is unimolecular Elementary steps involving the collision of two reactant molecules are bimolecular Elementary steps involving the simultaneous collision of three molecules are termolecular The chance that an elementary step involves the simultaneous collision of 4 molecules is extremely remote. Multistep Mechanisms A multistep mechanism consists of a sequence of elementary steps. For example: NO2 + CO → NO + CO2 Actually occurs in two steps 1st step – two NO2 molecules collide to transfer one O atom NO2 + NO2 → NO + NO3 2nd step- the NO3 collides with CO and transfers an O atom NO3 + CO → NO2 + CO2 The elementary steps always add together to give the overall chemical equation NO2 + NO2 → NO + NO3 NO3 + CO → NO2 + CO2 2NO2 + NO3 +CO → NO2 + NO3 + CO2 + NO NO2 + CO → NO + CO2 If a reactant cancels so that it is neither a reactant or a product in the overall process it is an intermediate • • • • Sample exercise 14.12 O 3 → O2 + O O3 + O → 2 O 2 1st step involves a single reactant so it is ________________ • 2O3→ 3O2 • O is the ___________ • • • • Sample exercise 14.12 O 3 → O2 + O O3 + O → 2 O 2 1st step involves a single reactant so it is unimolecular • 2O3→ 3O2 • O is the intermediate Work practice exercise Rate Laws for Elementary Steps Rate laws must be determined experimentally and cannot be predicted from coefficients of balanced equations The rate law can be determined from the mechanism If we know the elementary steps of a rxn then we know the rate law Elementary Steps and Rate Laws Molecularity Elementary steps Rate Law Unimolecular A→products rate = k[A] Bimolecular A + A→products rate = k [A]2 Bimolecular A+B→products rate = k[A][B] Termolecular A+A+A→products rate = k[A]3 Termolecular A+A+B→products rate = k[A]2[B] Termolecular A+B+C→products rate= k[A][B][C] The elementary step of a rxn determines the rate, not the overall rxn. Therefore, we cannot determine rate from the coefficients Exercise 14.13 most rxns involve more than one elementary step often one step is much slower than the others b/c the slow step limits the overall rxn, it is called the rate determining step or the rate limiting step. Each step has its own Ea and rate constant. The rate determining step governs the rate law for the overall rxn. For example: NO2 + CO → NO + CO2 Experimentally we know is second order in NO2 and zero order in CO. The proposed rxn mechanism is k1 NO2 + NO2 → NO + NO3 (slow) k2 NO3 + CO → NO2 + CO2 (fast) Rate = k1[NO2]2 we know that the rate law cannot be rate = k[NO2][CO] b/c changing the conc of CO experimentally does not change the rate of reaction. Mechanisms with an initial fast step (for example) 2NO + Br2 → 2NOBr experimentally the rate law has been determined to be rate= k[NO]2[Br2] we would expect a single termolecular step: NO + NO + Br2→ 2NOBr rate = k[NO]2[Br2] However , termolecular processes are extremely rare. The actual process has the first step as the fast step and the second step as the slow (rate determining ) step Step 1 ( fast) k1 NO + Br2 ↔NOBr2 Step 2 ( slow) k2 NOBr2 + NO → 2NOBr It is difficult to determine the conc. of NOBr2 b/c it is an intermediate. Intermediates are unstable and tend to have a low conc. b/c the 1st step is fast and the second step is slow the [NOBr2] = [NO][Br2] so looking at step two : rate = k[NO][Br2][NO] = k [NO]2[Br2] whenever a fast step precedes a slow one, we can solve for the conc. of the intermediate by assuming that an equilibrium is established in the first step. Exercise 14 Section 7 Catalysis A catalyst lowers the overall activation energy for a chemical reaction. A catalyst is a substance that changes the speed of a chemical reaction without undergoing permanent change itself. A catalyst that is present in the same phase as the reacting molecules is a homogeneous catalyst A catalyst usually lowers Ea by providing a completely different rxn mechanism • A heterogeneous catalyst exists in a different phase than either of the reactants • Heterogenous catalysts are often made of metals or metal oxides The initial step of heterogeneous catalysts is usually adsorption in which the molecules bind to the surface. Adsorption usually happens b/c the atoms at the surface are extremely reactive. The places where the reacting molecules become adsorbed are called active sites. The reacting molecules are in close proximity to one another increasing the probability of rxn. Catalytic converters are an example of heterogeneous catalysts. The job of the catalytic converter is to convert CO and CxHy( unused hydrocarbons) to CO2 and H2O and to convert NO and NO2 to N2 and O2 Transition metal oxides and noble metals are used for the catalytic converters Usually the catalysts that are the most effective for one rxn are much less effective for the other. It is necessary to have two compartments on the catalytic converter 35% of the Pt, 65% of the Pd and 95% of the Rh used annually is due to catalytic converters. All of these metals come from South Africa and Russia and are more expensive than gold. Biological catalysts- enzymes Enzymes tend to be very large proteins Enzymes are very selective for the reaction they catalyze The position on the molecule where the rxn occurs is called the active site The substance that undergoes rxn at the reaction site is the substrate Lock and Key Model The substrate fits neatly in to the active site on the enzyme As the substrate enters the active site it is somehow activated and capable of extreme rapid reaction. ( possibly loss of electron, change of molecular shape or distribution of the substrate bonds) • Once rxn occurs, the substrate leaves the bond allowing another substrate to enter. • An enzyme inhibitor will bond to the active sit and block the entry site The number of individual catalyzed reactions it called turnover number Enzymes have large turnover numbers meaning they have very low activation energy.