Kinetics
advertisement

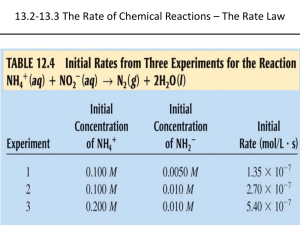
Kinetics/Equilibrium A unit dedicated to studying the rate of chemical reactions (how quickly reactions take place) Spontaneous processes • A spontaneous process is a chemical reaction in which a system releases free energy (most often as heat) and moves to a lower, more thermodynamically stable, energy state. – Examples: • Burning a log after igniting the wood. • Rusting • A ball falling through the air. What ultimately determines if a reaction is spontaneous or not? • ΔG = ΔH - TΔS ΔG = Change in Gibbs Free Energy ΔH = Enthalpy change T = temperature of the system (Kelvin) ΔS = entropy change If ΔG is negative, the reaction is spontaneous. Factors affecting spontaneity? • When ΔS is positive and ΔH is negative, a process is spontaneous • When ΔS is positive and ΔH is positive, a process is spontaneous at high temperatures, where exothermicity plays a small role in the balance. • When ΔS is negative and ΔH is negative, a process is spontaneous at low temperatures, where exothermicity is important. • When ΔS is negative and ΔH is positive, a process is not spontaneous at any temperature, but the reverse process is spontaneous. Examples of entropy changes – you decide?? • Phases changes from solids liquids gases? • Chemical reactions in which the moles of gases produced is greater than the moles of gases used? Example: C(s) + O2(g) 2CO(g) • Dissolving a solid into a liquid solvent to produce ions? • Dissolving a gas into a liquid solvent? • Molecular complexity? NaCl CaCl2 AlCl3 Homework: • Read Chapter 8 – section 1 • Answer questions on pages 262-263, questions 2,4,6,8,9 Reaction rates • The rate of a reaction is the same as the rate of any other process – it is a measure of the change in something over time. – For a moving object, rate (speed) is the change in the position of the object over time. – For reactions, it is a change in the concentration of something over time. What does the plot of reaction rate vs. time look like? NOTE: What happens to the rate of a reaction as the concentration of the reactants decreases? NOTE: What happens to the concentration of the reactants as the reaction proceeds? To find Instantaneous rate at any given point of the reaction, simply… • Find the tangent of the curve at any given point on the plot of reactant concentration vs. time. Factors that effect the rate of a reaction: • 1) The initial concentration of the reactants (the greater the concentration, the faster the rate of the reaction.) • 2) The presence of a catalyst (A catalyst is something that lowers the activation energy of the reaction, and therefore speeds up the rate of the reaction.) • 3) The temperature of the reactants (at higher temperatures, the reactants are moving faster and are at a higher energy state, so there is a greater rate which collisions result in products. • 4) The surface area of the reactants (The greater the surface area, the faster the reaction.) What theory explains these factors? • "collision theory" - which states that for molecules to react, they must: – collide – have the right energy – have the right geometry To increase the rate, you must make the above more likely to occur. This is possible by changing other factors such as: 1) increasing the surface area (of solids)-this allows for more collisions and gives more molecules the right geometry 2) increasing the temperature-this gives more molecules the right energy (also called the activation energy, Ea) 3) increasing the concentration (of gases and solutions)-this allows for more collisions and more correct geometry 4) using a catalyst-helps molecules achieve the correct geometry by providing a different way to react How do catalysts speed up reactions??? • Catalysts move the energy barrier to the left as noted earlier – or, more significantly, they lower the activation energy of the reaction. Rate Laws? • for the general reaction of A and B to give products E and F: • a A + b B --> e E + f F (where a, b, e, and f are stoichiometric coefficients) the rate is defined as • R = -d[A]/a dt = -d[B]/b dt = + d[E]/e dt = + d[F]/f dt • Thus for the general reaction of A and B considered to the left, we might find • R = -= k [A]a[B]b where the exponents a and b need not be integers or half-integers and are not necessarily equal to the stoichiometric coefficients a and b. Can you arrive at the correct rate law expression? • Example Problem: Find the rate law and rate constant of A + B --> C using the following data Another example!! • • • • • How do we determine the rate law from initial rates? [A] 0.100 0.100 0.200 [B] 0.100 0.200 0.100 Initial rate 4 x 10 -5 M/s 4 x 10 -5 M/s 1.6 x 10 -4 M/s The rate law for this data is: Rate = k [A]2[B]0 • The general rate law for any reaction is: Rate = k [reactant 1]m[reactant 2]n With m & n the reaction orders for each reactant with their sum the overall reaction order. What is meant by the order of a reaction? • Reaction order is a topic which comes with reaction rates. If you have a reaction in which A, B, and C are possible reactants, then we can describe the order of the reaction following this chart. Homework??? • Complete the worksheet given in class. What is meant by overall reaction molecularity? consider the following reaction: NO(g) + O3(g) NO2(g) + O2(g) • If NO molecules collide with ozone molecules, we will produce nitrogen dioxide and oxygen gas. (Note: This equation shows the decay of ozone from nitrous oxide – therefore NO is an ozone depleting gas!!) The previous reaction is bimolecular – (a result of a collision of 2 reactant molecules) New term – molecularity – the number of molecules that participates as reactants in the elementary step of a reaction. Example: The decomposition of a peroxide into oxygen and water would be unimolecular. Example: reactions involving the simultaneous collisions of three molecules would be called termolecular (very rare – it is hard to get three molecules to collide with the right orientation) Summarize: A products A + A products A + B products A + A + A products A + A + B products A + B + C products unimolecular bimolecular bimolecular termolecular termolecular termolecular The previous reaction is called an elementary process (because it occurs in one elementary step) – Not all reactions are elementary processes. • Consider: NO2(g) + CO(g) NO(g) + CO2(g) The actual reaction is the following: NO2 + NO2 NO3 + NO NO3 + CO NO2 + CO2 • Note: If we add these two elementary steps up, we get the above net reaction. • NO3 would be called an intermediate – it is used up as soon as it is formed. • Also, it is important to note that each of the elementary steps has there own rate constant and there own rate law. The slower step would be the rate determining step. How do we know a reaction mechanism is needed to explain the reaction??? • Any time the rate law expression generated from the balanced equation does NOT agree with what is observed through experimentation, you need to consider a reaction mechanism!! Homework??? • Complete the worksheet given in class!! A cool on line lab!! • Lab #1 – persulfate ion and iodide ion reaction