pptx
advertisement
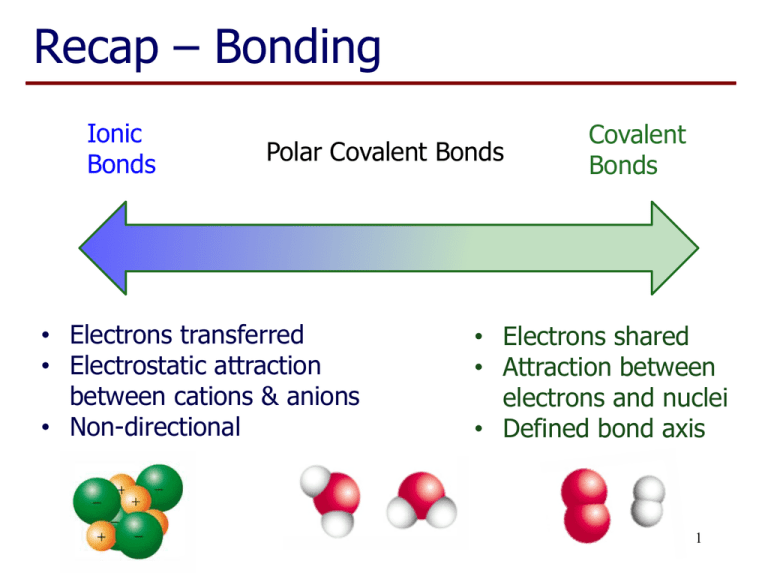
Recap – Bonding Ionic Bonds Polar Covalent Bonds • Electrons transferred • Electrostatic attraction between cations & anions • Non-directional Covalent Bonds • Electrons shared • Attraction between electrons and nuclei • Defined bond axis 1 Metallic Bonding • Outer electrons of a metal atom are not fixed to that atom but spread around surrounding atoms or ‘delocalized’. • Malleable and ductile – atoms able to slide past each other in a “sea of electrons”. • Mobile electrons – good electrical and thermal conductivity in solid and molten state. 2 Types of Formula Empirical formula • Lowest ratio of atoms of different types present. • • • Ionic Compounds, eg NaCl, K2O Covalent compounds, eg glucose CH2O Elements, always used except for diatomic elements (H2, N2, O2, F2, Cl2, Br2, I2) 3 Formulae for Molecules Molecular formula: • Actual number of atoms of each type in molecule. Eg. Glucose has molecular formula C6H12O6. • Implies covalent bonding. • Always a simple multiple of empirical formula. Eg. Empirical formula: Molecular formula: CH C2H2 (ethyne) C6H6 (benzene) 4 Formulae for Molecules Structural formula: • Shows connectivity of atoms. • Eg glucose Empirical: CH2O Molecular: C6H12O6 • Eg hydrogen peroxide Empirical: HO Molecular: H2O2 5 Allotropes • Allotropes: Elements with different bonding diamond Toby Hudson (School of Chemistry, University of Sydney) Example: Carbon commons.wikimedia.org/wiki/File:Apollo_synthetic_diamond.jpg arrangement of atoms. • Usually one form is more stable at a given temperature and pressure. graphite buckminsterfullerene nanotube 6 Molecular vs Network Solids Covalent bonded substances maybe ‘molecular’ or ‘network’ materials. • Eg the elements iodine and carbon The purple colour of the vapour above solid iodine is due to I2 molecules in the gas phase. 7 Molecular vs Network Solids Molecular materials are: • Soft when solid (eg wax) with a low melting point • May also be gases or liquids at room temperature • Eg CO2 Network materials are: • Hard solids with a high melting point • Covalent bonds broken when solid melts • Eg SiO2 8 Learning Outcomes: • By the end of this lecture, you should: − − − − − − understand the basis of bonding in metals appreciate the differences between empirical, molecular and structural formulae know the context in which the different types of formula are used (elements, ionic, covalent compounds) know what an allotrope is understand the different characteristics of a network and molecular solids be able to complete the worksheet (if you haven’t already done so…) 9 Questions to complete for next lecture: 1. When a material is heated the energy provided results in increased motion of the particles present. Why are metals good conductors of heat? 2. Name the two allotropes of the element with atomic number 8. 3. Silicon has a melting point of 1410 C and phosphorus has a melting point of 44 C. What does this suggest about the nature of bonding in the two solids? 4. Silicon carbide (SiC) is a solid at temperatures up to about 3000 C. What does this suggest about the type of structure of this material? 10




